CRESEMBA Drug Patent Profile
✉ Email this page to a colleague
When do Cresemba patents expire, and when can generic versions of Cresemba launch?
Cresemba is a drug marketed by Astellas and is included in two NDAs. There are three patents protecting this drug.
This drug has thirty-two patent family members in nineteen countries.
The generic ingredient in CRESEMBA is isavuconazonium sulfate. One supplier is listed for this compound. Additional details are available on the isavuconazonium sulfate profile page.
DrugPatentWatch® Generic Entry Outlook for Cresemba
Cresemba was eligible for patent challenges on March 6, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 8, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for CRESEMBA
| International Patents: | 32 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 34 |
| Clinical Trials: | 5 |
| Drug Prices: | Drug price information for CRESEMBA |
| What excipients (inactive ingredients) are in CRESEMBA? | CRESEMBA excipients list |
| DailyMed Link: | CRESEMBA at DailyMed |
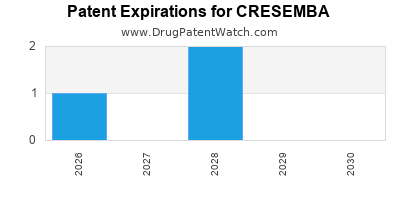
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for CRESEMBA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for CRESEMBA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Pfizer | Phase 4 |
| Jeffrey Jenks, MD, MPH | Phase 3 |
| Astellas Pharma Global Development, Inc. | Phase 3 |
Pharmacology for CRESEMBA
| Drug Class | Azole Antifungal |
| Mechanism of Action | Cytochrome P450 3A4 Inhibitors Organic Cation Transporter 2 Inhibitors P-Glycoprotein Inhibitors |
US Patents and Regulatory Information for CRESEMBA
CRESEMBA is protected by three US patents and fifteen FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of CRESEMBA is ⤷ Sign Up.
This potential generic entry date is based on TREATMENT OF INVASIVE ASPERGILLOSIS IN PEDIATRIC PATIENTS 6 YEARS OF AGE AND OLDER WHO WEIGH 16 KILOGRAMS (KG) AND GREATER.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting CRESEMBA
Active ingredient containing stabilised solid forms and method for the production thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Active ingredient containing stabilised solid medicinal forms and methods for the production thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
N-substituted carbamoyloxyalkyl-azolium derivatives
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting CRESEMBA
TREATMENT OF INVASIVE ASPERGILLOSIS IN PEDIATRIC PATIENTS 6 YEARS OF AGE AND OLDER WHO WEIGH 16 KILOGRAMS (KG) AND GREATER
Exclusivity Expiration: ⤷ Sign Up
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
FDA HAS NOT RECOGNIZED ORPHAN-DRUG EXCLUSIVITY (ODE) FOR THIS DRUG, BUT IT CONTAINS THE SAME ACTIVE MOIETY OR MOIETIES AS ANOTHER DRUG(S) THAT WAS ELIGIBLE FOR ODE, AND ALSO SHARES ODE-PROTECTED USE(S) OR INDICATION(S) WITH THAT DRUG(S).AN APPLICATION SEEKING APPROVAL FOR THE SAME ACTIVE MOIETY OR MOIETIES, INCLUDING AN ANDA THAT CITES THIS NDA AS ITS BASIS OF SUBMISSION, MAY NOT BE APPROVED FOR SUCH ODE-PROTECTED USE(S) AND INDICATION(S)
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF INVASIVE MUCORMYCOSIS IN PEDIATRIC PATIENTS 6 YEARS OF AGE AND OLDER WHO WEIGH 16 KG AND GREATER
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
GENERATING ANTIBIOTIC INCENTIVES NOW
Exclusivity Expiration: ⤷ Sign Up
GENERATING ANTIBIOTIC INCENTIVES NOW
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF INVASIVE MUCORMYCOSIS IN PATIENTS 18 YEARS OF AGE AND OLDER
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF INVASIVE ASPERGILLOSIS
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF INVASIVE MUCORMYCOSIS IN PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF INVASIVE ASPERGILLOSIS IN PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astellas | CRESEMBA | isavuconazonium sulfate | POWDER;INTRAVENOUS | 207501-001 | Mar 6, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Astellas | CRESEMBA | isavuconazonium sulfate | CAPSULE;ORAL | 207500-001 | Mar 6, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Astellas | CRESEMBA | isavuconazonium sulfate | POWDER;INTRAVENOUS | 207501-001 | Mar 6, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Astellas | CRESEMBA | isavuconazonium sulfate | CAPSULE;ORAL | 207500-001 | Mar 6, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Astellas | CRESEMBA | isavuconazonium sulfate | CAPSULE;ORAL | 207500-001 | Mar 6, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Astellas | CRESEMBA | isavuconazonium sulfate | CAPSULE;ORAL | 207500-002 | Nov 22, 2022 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for CRESEMBA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astellas | CRESEMBA | isavuconazonium sulfate | CAPSULE;ORAL | 207500-001 | Mar 6, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Astellas | CRESEMBA | isavuconazonium sulfate | POWDER;INTRAVENOUS | 207501-001 | Mar 6, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for CRESEMBA
When does loss-of-exclusivity occur for CRESEMBA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 07302320
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 59342
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 63941
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 68842
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 02708
Estimated Expiration: ⤷ Sign Up
Patent: 68842
Estimated Expiration: ⤷ Sign Up
Germany
Patent: 2007003037
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering CRESEMBA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2019524030 | 新しい無線のためのアップロード制御シグナリング | ⤷ Sign Up |
| Japan | 3787307 | ⤷ Sign Up | |
| China | 113965295 | 新无线电中的物理信道 (Physical channels in new radio) | ⤷ Sign Up |
| South Korea | 20220141916 | 새로운 라디오 다운링크 제어 채널 (NEW RADIO DOWNLINK CONTROL CHANNEL) | ⤷ Sign Up |
| South Korea | 20180129954 | 다운링크 동기화 | ⤷ Sign Up |
| Germany | 60018989 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for CRESEMBA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1280795 | CR 2016 00002 | Denmark | ⤷ Sign Up | PRODUCT NAME: ISAVUCONAZOLE AS ISAVUCONAZONIUM SULFATE OR AS AN ISAVUCONAZONIUM SALT WITH ANY OTHER PHARMACEUTICALLY ACCEPTABLE ANION, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE, OR SOLVATE; REG. NO/DATE: EU/1/15/1036 20151019 |
| 1280795 | 512 | Finland | ⤷ Sign Up | |
| 1280795 | 300791 | Netherlands | ⤷ Sign Up | PRODUCT NAME: ISAVUCONAZOL ALS ISAVUCONAZONIUM SULFAAT OF ALS EEN ISAVUCONAZONIUM ZOUT MET ELK ANDER FARMACEUTISCH AANVAARDBAAR ANION, NAAR KEUZE IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, HYDRAAT OF SOLVAAT; REGISTRATION NO/DATE: EU/1/15/1036 20151019 |
| 1280795 | 1690011-0 | Sweden | ⤷ Sign Up | PRODUCT NAME: ISAVUCONAZOLE; REG. NO/DATE: EU/1/15/1036 20151016 |
| 1280795 | CA 2016 00002 | Denmark | ⤷ Sign Up | PRODUCT NAME: ISAVUCONAZOLE AS ISAVUCONAZONIUM SULFATE OR AS AN ISAVUCONAZONIUM SALT WITH ANY OTHER PHARMACEUTICALLY ACCEPTABLE ANION, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE, OR SOLVATE; REG. NO/DATE: EU/1/15/1036 20151019 |
| 1280795 | 122016000011 | Germany | ⤷ Sign Up | PRODUCT NAME: ISAVUCONAZONIUM ALS ISAVUCONAZONIUM-SULFAT ODER ALS SALZ MIT EINEM ANDEREN PHARMAZEUTISCH VERTRAEGLICHEN ANION, SOWIE HYDRATE ODER SOLVATE DAVON; REGISTRATION NO/DATE: EU/1/15/1036 20151015 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


