CHANTIX Drug Patent Profile
✉ Email this page to a colleague
When do Chantix patents expire, and when can generic versions of Chantix launch?
Chantix is a drug marketed by Pf Prism Cv and is included in one NDA.
The generic ingredient in CHANTIX is varenicline tartrate. There are twelve drug master file entries for this compound. Twenty-two suppliers are listed for this compound. Additional details are available on the varenicline tartrate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Chantix
A generic version of CHANTIX was approved as varenicline tartrate by PAR PHARM INC on August 11th, 2021.
Summary for CHANTIX
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 98 |
| Clinical Trials: | 166 |
| Patent Applications: | 155 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for CHANTIX |
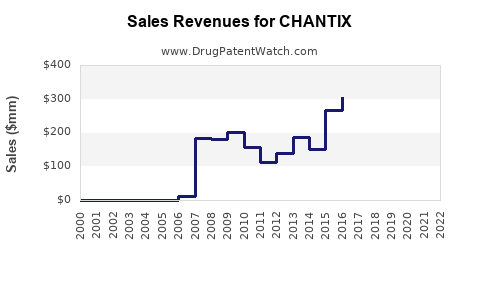
| Drug Sales Revenues: | Drug sales revenues for CHANTIX |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for CHANTIX |
| What excipients (inactive ingredients) are in CHANTIX? | CHANTIX excipients list |
| DailyMed Link: | CHANTIX at DailyMed |
Recent Clinical Trials for CHANTIX
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Massachusetts General Hospital | Phase 3 |
| Mayo Clinic | Early Phase 1 |
| Duke University | Phase 4 |
Pharmacology for CHANTIX
| Drug Class | Cholinergic Receptor Agonist Partial Cholinergic Nicotinic Agonist |
| Mechanism of Action | Cholinergic Agonists Partial Cholinergic Nicotinic Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for CHANTIX
Paragraph IV (Patent) Challenges for CHANTIX
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| CHANTIX | Tablets | varenicline tartrate | 0.5 mg and 1 mg | 021928 | 5 | 2010-05-10 |
US Patents and Regulatory Information for CHANTIX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pf Prism Cv | CHANTIX | varenicline tartrate | TABLET;ORAL | 021928-001 | May 10, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pf Prism Cv | CHANTIX | varenicline tartrate | TABLET;ORAL | 021928-002 | May 10, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for CHANTIX
See the table below for patents covering CHANTIX around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Eurasian Patent Organization | 005316 | АРИЛКОНДЕНСИРОВАННЫЕ АЗАПОЛИЦИКЛИЧЕСКИЕ СОЕДИНЕНИЯ (ARYL FUSED AZAPOLYCYCLIC COMPOUNDS) | ⤷ Try a Trial |
| Japan | 2003524002 | ⤷ Try a Trial | |
| Norway | 20024042 | ⤷ Try a Trial | |
| Hungary | 0100949 | ⤷ Try a Trial | |
| Bulgaria | 65891 | ⤷ Try a Trial | |
| European Patent Office | 1259489 | COMPOSES AZAPOLYCYCLIQUES A FUSION ARYLE (ARYL FUSED AZAPOLYCYCLIC COMPOUNDS) | ⤷ Try a Trial |
| Bulgaria | 104561 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for CHANTIX
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1044189 | CA 2008 00031 | Denmark | ⤷ Try a Trial | |
| 1044189 | SPC021/2008 | Ireland | ⤷ Try a Trial | SPC021/2008: 20081105, EXPIRES: 20210925 |
| 1044189 | 91442 | Luxembourg | ⤷ Try a Trial | 91442, EXPIRES: 20210926 |
| 1044189 | SPC/GB08/034 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: VARENICLINE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, INCLUDING THE TARTRATE SALT; REGISTRATION NO/DATE: EU/1/06/360/001 - 010 20060928 |
| 1044189 | 325 | Finland | ⤷ Try a Trial | |
| 1044189 | 300355 | Netherlands | ⤷ Try a Trial | 300355, 20181113, EXPIRES: 20220425 |
| 1044189 | PA2008010 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: VARENICLINUM TARTRAT; REG. NO/DATE: EU/1/06/360/001-010 20060926 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


