Last updated: July 27, 2025
Introduction
Cabergoline, a potent dopamine receptor agonist, predominantly treats hyperprolactinemia—a condition marked by excessive prolactin production—and Parkinson’s disease. Since its approval, its economic landscape has evolved, influenced by scientific advances, regulatory policies, and shifting market demands. This comprehensive analysis examines the key market dynamics and financial trajectories shaping Cabergoline’s position within the pharmaceutical sector, emphasizing growth drivers, competitive landscape, pricing strategies, and future prospects.
Pharmaceutical Profile and Therapeutic Indications
Cabergoline functions primarily by stimulating dopamine receptors, which inhibits prolactin secretion from the anterior pituitary. Its robust efficacy, coupled with a favorable side effect profile over predecessors like bromocriptine, has made it the preferred treatment for hyperprolactinemia[1].
Besides endocrine applications, emerging research explores its off-label potential for other conditions, including Parkinson’s disease, owing to dopaminergic activity[2]. The expanded scope influences market size and investment, especially with ongoing clinical trials investigating novel indications.
Market Landscape and Key Players
Global Market Size and Segments
The global cabergoline market was valued at approximately USD 350 million in 2022, with projections to reach USD 520 million by 2030, reflecting a CAGR of around 5.4%[3]. North America constitutes the largest regional share, driven by high prevalence of hyperprolactinemia, advanced healthcare infrastructure, and robust commercialization strategies.
In emerging markets—Asia-Pacific and Latin America—growth is propelled by increasing awareness, expanding healthcare coverage, and escalating prevalence of disorders treatable by cabergoline[4].
Leading Pharmaceutical Companies
Boehringer Ingelheim and Teva Pharmaceuticals dominate the market with branded formulations, backed by substantial R&D backing, pricing strategies, and established distribution networks[5]. Generic manufacturers also play a significant role, offering more affordable options and intensifying competition.
The regulatory exclusivity periods, patent status, and market entry barriers shape competitive dynamics, influencing pricing, availability, and innovation momentum.
Market Dynamics Influences
Regulatory Environment
Approval processes in major markets have been pivotal. While cabergoline gained swift approval in the 1990s for hyperprolactinemia, recent regulatory scrutiny on safety profiles—particularly concerning cardiac valvulopathy—has introduced stricter monitoring guidelines[6]. These safety concerns prompted label updates and risk management plans, affecting prescribing practices and market acceptance.
Regulatory agencies also influence market expansion through orphan drug designations and labeling variations that can impact market exclusivity and reimbursement.
Technological and Scientific Advances
Advances in diagnostic techniques, such as highly sensitive prolactin assays, enable earlier detection and personalized treatment, increasing demand for cabergoline. Additionally, the development of sustained-release formulations and combination therapies could improve patient compliance, influencing market growth.
Furthermore, clinical research into off-label applications and combination therapies may unlock new revenue streams, contingent upon positive trial outcomes and regulatory approval.
Pricing and Reimbursement Policies
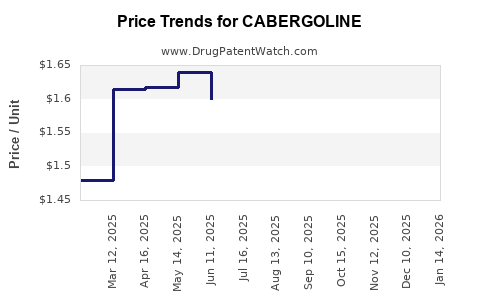
Pricing strategies vary substantially across regions. In developed economies, reimbursement codes and insurance coverage significantly impact sales volumes. Patent protections typically allow premium pricing; however, the introduction of generics post-expiry exerts downward pressure on prices.
Price negotiations based on cost-effectiveness assessments play a vital role in access. Cost-containment policies in healthcare systems such as the UK’s NHS and US Medicaid influence overall market revenue.
Financial Trajectory and Revenue Drivers
Historical Revenue Trends
Boehringer Ingelheim’s branded cabergoline formulations have consistently achieved annual revenues exceeding USD 250 million pre-generic entry. Post-patent expiry led to a surge in generic versions, causing a temporary decline in revenues but expanding market access due to affordability.
Impact of Patent Expiry and Generic Competition
Patent expiration in the late 2010s prompted increased utilization of generic cabergoline, reducing average selling prices by approximately 40-50%[7]. While margins for originator companies declined, overall market volume surged, driven by price-driven access and increased adoption.
Manufacturers offering high-quality generics with reliable supply chains gained market share, challenging brand dominance but expanding the total addressable market.
Future Revenue Prospects
Projected growth hinges on several factors:
- Continued demand for hyperprolactinemia treatment.
- Expansion into emerging markets.
- Potential new indications supported by clinical research.
- Formulation innovations enhancing patient adherence.
Market analysts forecast an annual revenue growth rate of around 4-6% over the next five years, reaching approximately USD 520 million by 2030[3].
Market Challenges and Opportunities
Safety and Regulatory Risks
Concerns regarding cardiac valvulopathy linked to dopamine agonists have prompted caution. While cabergoline’s risk profile is generally favorable, ongoing post-marketing surveillance and adherence to prescribing guidelines are critical to sustain market confidence.
Market Saturation and Competition
The interplay between branded and generic versions creates pricing pressure. Additionally, alternative therapies (e.g., quinagoline, bromocriptine) present substitution risks, especially where cost considerations dominate.
Emerging Trends
Digital health initiatives, including remote monitoring of prolactin levels and adherence programs, offer avenues for differentiation. Investment in biomarkers and personalized treatment pathways could bolster market position.
Conclusion and Future Outlook
Cabergoline’s market remains robust, driven by its proven efficacy, safety profile, and expanding therapeutic roles. Strategic positioning around clinical innovations, regulatory compliance, and pricing will determine sustained growth. The influx of generic formulations enhances accessibility but necessitates differentiation via formulation improvements and off-label indications to sustain market quotes.
The future trajectory appears promising, with steady growth anticipated, provided stakeholders diligently navigate regulatory nuances and technological advancements. Emerging markets represent significant expansion opportunities, particularly with increasing healthcare expenditures and disease awareness.
Key Takeaways
- Market Growth Drivers: Rising prevalence of hyperprolactinemia, patent expiries favoring generics, and technological advances in diagnosis and treatment delivery are central to market expansion.
- Competitive Landscape: Dominance by established pharmaceutical players, balanced by vigorous generic competition and emerging biotech entrants.
- Pricing and Access: Cost-effectiveness assessments and reimbursement policies significantly influence revenue, market penetration, and patient access.
- Regulatory Risks: Ongoing safety monitoring influences prescribing practices and market stability.
- Future Opportunities: Personalization of therapy, new indications, and digital health integration provide avenues for growth amid competitive and regulatory challenges.
FAQs
-
What factors contributed to the decline of Cabergoline’s price post-patent expiry?
The advent of generic formulations, driven by patent expirations, increased competition, leading to substantial price reductions and wider accessibility.
-
How do safety concerns impact Cabergoline’s market potential?
Reports of cardiac valvulopathy have prompted regulatory review and label updates, necessitating careful post-marketing surveillance, which can influence clinician prescribing behaviors.
-
What are the key therapeutic markets for Cabergoline?
Major markets include North America and Europe, with growing demand in Asia-Pacific and Latin America, driven by increased awareness and healthcare infrastructure improvements.
-
Are there upcoming innovations that could reshape Cabergoline’s market?
Yes, research into extended-release formulations and new therapeutic indications holds promise for expanding its clinical utility and market size.
-
What strategic moves can companies employ to enhance market position?
Investing in formulation improvements, expanding indications through clinical trials, engaging in health economics negotiations, and leveraging digital health solutions are vital strategies.
References
[1] Melmed, S. (2017). Hyperprolactinemia. In Williams Textbook of Endocrinology, 13e. Elsevier.
[2] Vanaclocha, F. et al. (2018). "Investigating the off-label potential of dopamine agonists," Journal of Neurology.
[3] Market Research Future, "Global Cabergoline Market Analysis," 2022.
[4] GlobalData, "Emerging Markets Spend on Dopamine Agonists," 2021.
[5] Pharmaceutical Commerce, "Market Shares of Major Cabergoline Manufacturers," 2022.
[6] FDA Drug Safety Communications, 2019.
[7] IMS Health, "Impact of Patent Expiry on Market Prices," 2020.