BOSULIF Drug Patent Profile
✉ Email this page to a colleague
When do Bosulif patents expire, and when can generic versions of Bosulif launch?
Bosulif is a drug marketed by Pf Prism Cv and is included in two NDAs. There are five patents protecting this drug and two Paragraph IV challenges.
This drug has eighty-one patent family members in thirty countries.
The generic ingredient in BOSULIF is bosutinib monohydrate. There are five drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the bosutinib monohydrate profile page.
DrugPatentWatch® Generic Entry Outlook for Bosulif
Bosulif was eligible for patent challenges on September 4, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 26, 2030. This may change due to patent challenges or generic licensing.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for BOSULIF
| International Patents: | 81 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 120 |
| Clinical Trials: | 16 |
| Patent Applications: | 4,099 |
| Drug Prices: | Drug price information for BOSULIF |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BOSULIF |
| What excipients (inactive ingredients) are in BOSULIF? | BOSULIF excipients list |
| DailyMed Link: | BOSULIF at DailyMed |
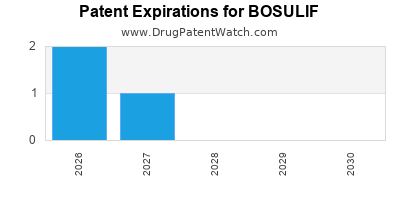

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BOSULIF
Generic Entry Dates for BOSULIF*:
Constraining patent/regulatory exclusivity:
TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH CHRONIC PHASE (CP) PHILADELPHIA CHROMOSOME-POSITIVE CHRONIC MYELOGENOUS LEUKEMIA (PH+ CML), NEWLY-DIAGNOSED OR RESISTANT OR INTOLERANT TO PRIOR THERAPY NDA:
Dosage:
TABLET;ORAL |
Generic Entry Dates for BOSULIF*:
Constraining patent/regulatory exclusivity:
TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH CHRONIC PHASE (CP) PHILADELPHIA CHROMOSOME-POSITIVE CHRONIC MYELOGENOUS LEUKEMIA (PH+ CML), NEWLY-DIAGNOSED OR RESISTANT OR INTOLERANT TO PRIOR THERAPY NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BOSULIF
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| City of Hope Medical Center | Phase 1 |
| Fundacion Espanola para la Curacion de la Leucemia Mieloide Cronica | Phase 1/Phase 2 |
| Roche Farma, S.A | Phase 1/Phase 2 |
Pharmacology for BOSULIF
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Bcr-Abl Tyrosine Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for BOSULIF
Paragraph IV (Patent) Challenges for BOSULIF
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BOSULIF | Tablets | bosutinib monohydrate | 400 mg | 203341 | 1 | 2018-10-25 |
| BOSULIF | Tablets | bosutinib monohydrate | 100 mg and 500 mg | 203341 | 2 | 2016-09-06 |
US Patents and Regulatory Information for BOSULIF
BOSULIF is protected by five US patents and seven FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of BOSULIF is ⤷ Sign Up.
This potential generic entry date is based on TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH CHRONIC PHASE (CP) PHILADELPHIA CHROMOSOME-POSITIVE CHRONIC MYELOGENOUS LEUKEMIA (PH+ CML), NEWLY-DIAGNOSED OR RESISTANT OR INTOLERANT TO PRIOR THERAPY.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting BOSULIF
Treatment of imatinib resistant leukemia
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
4-anilino-3-quinolinecarbonitriles for the treatment of chronic myelogenous leukemia (CML)
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline forms of 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-pipera- zinyl)propoxy]-3-quinolinecarbonitrile and methods of preparing the same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
4-anilino-3-quinolinecarbonitriles for the treatment of chronic myelogenous leukemia (CML)
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Substituted 3-cyanoquinolines
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting BOSULIF
TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH CHRONIC PHASE (CP) PHILADELPHIA CHROMOSOME-POSITIVE CHRONIC MYELOGENOUS LEUKEMIA (PH+ CML), NEWLY-DIAGNOSED OR RESISTANT OR INTOLERANT TO PRIOR THERAPY
Exclusivity Expiration: ⤷ Sign Up
NEW PRODUCT
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF ADULT PATIENTS WITH NEWLY-DIAGNOSED CHRONIC PHASE (CP) PHILADELPHIA CHROMOSOME-POSITIVE CHRONIC MYELOGENOUS LEUKEMIA (PH+ CML)
Exclusivity Expiration: ⤷ Sign Up
FOR THE TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH CHRONIC PHASE (CP) PHILADELPHIA CHROMOSOME-POSITIVE CHRONIC MYELOGENOUS LEUKEMIA (PH+ CML), NEWLY-DIAGNOSED OR RESISTANT OR INTOLERANT TO PRIOR THERAPY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-002 | Sep 4, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-003 | Oct 27, 2017 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-002 | Sep 4, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-002 | Sep 4, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-002 | Sep 4, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-001 | Sep 4, 2012 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-003 | Oct 27, 2017 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BOSULIF
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-002 | Sep 4, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-001 | Sep 4, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-003 | Oct 27, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BOSULIF
When does loss-of-exclusivity occur for BOSULIF?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4505
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 06266045
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0613574
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 13053
Estimated Expiration: ⤷ Sign Up
China
Patent: 1248047
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 96
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 14781
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 02029
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 078063
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 02029
Estimated Expiration: ⤷ Sign Up
Guatemala
Patent: 0600282
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 14614
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 09500332
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 08000384
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 080051
Estimated Expiration: ⤷ Sign Up
Panama
Patent: 85101
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 070190
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 02029
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 02029
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 07148072
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 02029
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 080028386
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 49197
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 0740797
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BOSULIF around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Costa Rica | 8350 | 4-ANILINO-3-QUINOLINCARBONITRILOS PARA EL TRATAMIENTO DE LEUCEMIA MIELOGENICA CRONICA | ⤷ Sign Up |
| Poland | 1902029 | ⤷ Sign Up | |
| Hong Kong | 1114614 | -二氯- -甲氧苯基 氨基 -甲氧基- -甲基- -哌嗪基 丙氧基 -喹啉腈之晶體形式及其之製備方法 (CRYSTALLINE FORMS OF 4-[(2,4-DICHLORO-5-METHOXYPHENYL)AMINO]-6-METHOXY-7- [3-(4-METHYL-1-PIPERAZINYL)PROPOXY]-3-QUINOLINECARB-ONITRILE AND METHODS OF PREPARING THE SAME 4-[(24--5-)]-6--7-[3-(4--1-)]-3-) | ⤷ Sign Up |
| Brazil | PI0416289 | 4-anilino-3-quinolinacarbonitrilas para o tratamento de leucemia mielógeno crÈnica (cml) | ⤷ Sign Up |
| South Korea | 20100017983 | TREATMENT OF IMATINIB RESISTANT LEUKEMIA USING 4-AMINOQUINOLINE-3-CARBONITRILES | ⤷ Sign Up |
| Canada | 2868899 | TRAITEMENT DE LEUCEMIE RESISTANT A L~IMATINIB EN UTILISANT DES 4-AMINOQUINOLINE-3-CARBONITRILES AYANT UNE MUTATION DANS LE GENE BCR-ABL (TREATMENT OF IMATINIB RESISTANT LEUKEMIA USING 4-AMINOQUINOLINE-3-CARBONITRILES HAVING MUTATION IN THE BCRABL GENE) | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2008150957 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BOSULIF
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1902029 | C20140016 00110 | Estonia | ⤷ Sign Up | PRODUCT NAME: BOSUTINIIB;REG NO/DATE: K(2013)1968 (LOPLIK) 02.04.2013 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


