BONJESTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Bonjesta, and what generic alternatives are available?
Bonjesta is a drug marketed by Duchesnay and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-five patent family members in thirty-two countries.
The generic ingredient in BONJESTA is doxylamine succinate; pyridoxine hydrochloride. There are fourteen drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the doxylamine succinate; pyridoxine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Bonjesta
A generic version of BONJESTA was approved as doxylamine succinate; pyridoxine hydrochloride by ACTAVIS LABS FL INC on August 19th, 2016.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BONJESTA?
- What are the global sales for BONJESTA?
- What is Average Wholesale Price for BONJESTA?
Summary for BONJESTA
| International Patents: | 65 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 4 |
| Clinical Trials: | 1 |
| Patent Applications: | 15 |
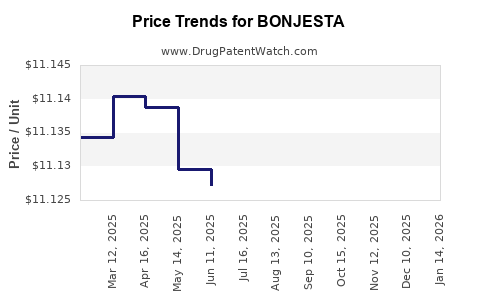
| Drug Prices: | Drug price information for BONJESTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BONJESTA |
| What excipients (inactive ingredients) are in BONJESTA? | BONJESTA excipients list |
| DailyMed Link: | BONJESTA at DailyMed |
Recent Clinical Trials for BONJESTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Duchesnay Inc. | Phase 3 |
| Health Decisions | Phase 3 |
Pharmacology for BONJESTA
| Ingredient-type | Analogs/Derivatives Vitamin B 6 |
| Drug Class | Antihistamine Vitamin B6 Analog |
| Mechanism of Action | Histamine Receptor Antagonists |
Paragraph IV (Patent) Challenges for BONJESTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BONJESTA | Extended-release Tablets | doxylamine succinate; pyridoxine hydrochloride | 20 mg/20 mg | 209661 | 1 | 2018-08-28 |
US Patents and Regulatory Information for BONJESTA
BONJESTA is protected by four US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BONJESTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BONJESTA
When does loss-of-exclusivity occur for BONJESTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0126
Patent: COMPOSICION DE DOXILAMINA Y PIRIDOXINA Y/O SUS METABOLITOS O SALES
Estimated Expiration: ⤷ Get Started Free
Patent: 2580
Patent: FORMA DE DOSIFICACIÓN DE DOXILAMINA Y PIRIDOXINA Y/O SUS METABOLITOS O SALES
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 13224598
Patent: Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014020186
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 48798
Patent: FORMULATION DE DOXYLAMINE ET DE PYRIDOXINE ET/OU DE LEURS METABOLITES OU SELS (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14001828
Patent: Sistema de dosificacion oral de liberacion dual que comprende doxilamina y piridoxina y/o sus analogos, metabolitos y sales de los mismos; forma de dosificacion oral de liberacion dual; kit farmaceutico; uso para aliviar los sintomas de nauseas y vomitos, incluidos los del embarazo.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4136004
Patent: Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 23122
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 26611
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 87971
Patent: FORMULATION DE DOXYLAMINE ET DE PYRIDOXINE ET/OU DE LEURS MÉTABOLITES OU SELS (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 26611
Patent: FORMULATION DE DOXYLAMINE ET DE PYRIDOXINE ET/OU DE LEURS MÉTABOLITES OU SELS (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 97035
Patent: 多西拉敏和吡哆醇和/或其代謝物或鹽的製劑 (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND OR METABOLITES OR SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 52301
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3644
Patent: צורות מינון בעלות שחרור דואלי הניתנות דרך הפה אשר מכילות דוקסילאמין ופירידוקסין, ערכות המכילות אותן ושימושן להקלת סימפטומים של בחילה והקאה (Dual release oral dosage forms comprising doxylamine and pyridoxine, kits comprising them and their use in alleviating the symptoms of nausea and vomiting)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 14701
Estimated Expiration: ⤷ Get Started Free
Patent: 15508082
Patent: ドキシラミンおよびピリドキシン、ならびに/またはその代謝物もしくは塩の製剤
Estimated Expiration: ⤷ Get Started Free
Patent: 16053092
Patent: ドキシラミンおよびピリドキシン、ならびに/またはその代謝物もしくは塩の製剤 (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 26611
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 5912
Patent: FORMULACIÓN DE DOXILAMINA Y PIRIDOXINA Y/O SUS METABOLITOS O SALES. (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14008594
Patent: FORMULACION DE DOXILAMINA Y PIRIDOXINA Y/O SUS METABOLITOS O SALES. (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7593
Patent: Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 26611
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 26611
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201403931Y
Patent: FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1588259
Estimated Expiration: ⤷ Get Started Free
Patent: 140139496
Patent: FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 09713
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 38657
Estimated Expiration: ⤷ Get Started Free
Patent: 1334780
Patent: Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 631
Patent: FORMULACIÓN DE DOXILAMINA Y PIRIDOXINA Y/O SUS METABOLITOS O SALES
Estimated Expiration: ⤷ Get Started Free
Patent: 283
Patent: FORMULACIÓN DE LIBERACIÓN PLURIMODAL DE DOXILAMINA Y PIRIDOXINA Y/O METABOLITOS O SALES DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BONJESTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Greece | 20030100286 | ΜΟΡΦΗΑΦΑΡΜΑΚΕΥΤΙΚΗΣΑΔΟΣΟΛΟΓΙΑΣΑΠΟΥΑΦΕΡΕΙΑΦΙΛΙΚΕΣΑΠΡΟΣΑΤΗΝΑΚΥΗΣΗΑΕΝΔΕΙΞΕΙΣ (PHARMACEUTICAL DOASAGE FORM BEARING PREGNANCY-FRIENDLY INDICIA) | ⤷ Get Started Free |
| Israel | 233644 | צורות מינון בעלות שחרור דואלי הניתנות דרך הפה אשר מכילות דוקסילאמין ופירידוקסין, ערכות המכילות אותן ושימושן להקלת סימפטומים של בחילה והקאה (Dual release oral dosage forms comprising doxylamine and pyridoxine, kits comprising them and their use in alleviating the symptoms of nausea and vomiting) | ⤷ Get Started Free |
| Cyprus | 1120788 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 2016029290 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BONJESTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3185856 | CR 2024 00001 | Denmark | ⤷ Get Started Free | PRODUCT NAME: COMBINATION OF DOXYLAMINE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AND PYRIDOXINE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; NAT. REG. NO/DATE: 66650 20230704; FIRST REG. NO/DATE: NL RVG 128835 20230216 |
| 3185856 | 122024000003 | Germany | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION AUS DOXYLAMIN, ODER EINES PHARMAZEUTISCH AKZEPTABLEN SALZES DAVON, UND PYRIDOXIN, ODER EINES PHARMZEUTISCH AKZEPTABLEN SALZES DAVON; NAT. REGISTRATION NO/DATE: 7006779.00.00 20230717; FIRST REGISTRATION: NIEDERLANDE RVG 128835 20230216 |
| 3185856 | CA 2024 00001 | Denmark | ⤷ Get Started Free | PRODUCT NAME: COMBINATION OF DOXYLAMINE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AND PYRIDOXINE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; NAT. REG. NO/DATE: 66650 20230704; FIRST REG. NO/DATE: NL RVG 128835 20230216 |
| 3185856 | 2024C/535 | Belgium | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE VAN DOXYLAMINE, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, EN PYRIDOXINE, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; AUTHORISATION NUMBER AND DATE: BE662396 20240321 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for BONJESTA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
