BEVESPI AEROSPHERE Drug Patent Profile
✉ Email this page to a colleague
When do Bevespi Aerosphere patents expire, and what generic alternatives are available?
Bevespi Aerosphere is a drug marketed by Astrazeneca and is included in one NDA. There are seven patents protecting this drug.
This drug has one hundred and ninety-two patent family members in thirty-two countries.
The generic ingredient in BEVESPI AEROSPHERE is formoterol fumarate; glycopyrrolate. There are nineteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the formoterol fumarate; glycopyrrolate profile page.
DrugPatentWatch® Generic Entry Outlook for Bevespi Aerosphere
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 28, 2030. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BEVESPI AEROSPHERE?
- What are the global sales for BEVESPI AEROSPHERE?
- What is Average Wholesale Price for BEVESPI AEROSPHERE?
Summary for BEVESPI AEROSPHERE
| International Patents: | 192 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 2 |
| Patent Applications: | 13 |
| Drug Prices: | Drug price information for BEVESPI AEROSPHERE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BEVESPI AEROSPHERE |
| What excipients (inactive ingredients) are in BEVESPI AEROSPHERE? | BEVESPI AEROSPHERE excipients list |
| DailyMed Link: | BEVESPI AEROSPHERE at DailyMed |
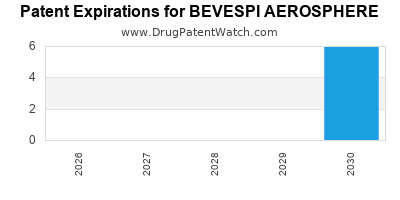
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BEVESPI AEROSPHERE
Generic Entry Date for BEVESPI AEROSPHERE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
AEROSOL, METERED;INHALATION |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BEVESPI AEROSPHERE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Bastiaan Driehuys | Phase 2/Phase 3 |
| Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center | Phase 4 |
| Los Angeles Biomedical Research Institute | Phase 4 |
Pharmacology for BEVESPI AEROSPHERE
US Patents and Regulatory Information for BEVESPI AEROSPHERE
BEVESPI AEROSPHERE is protected by seven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of BEVESPI AEROSPHERE is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,324,266.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | BEVESPI AEROSPHERE | formoterol fumarate; glycopyrrolate | AEROSOL, METERED;INHALATION | 208294-001 | Apr 25, 2016 | RX | Yes | Yes | 8,324,266 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Astrazeneca | BEVESPI AEROSPHERE | formoterol fumarate; glycopyrrolate | AEROSOL, METERED;INHALATION | 208294-001 | Apr 25, 2016 | RX | Yes | Yes | 8,815,258 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Astrazeneca | BEVESPI AEROSPHERE | formoterol fumarate; glycopyrrolate | AEROSOL, METERED;INHALATION | 208294-001 | Apr 25, 2016 | RX | Yes | Yes | 8,703,806 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for BEVESPI AEROSPHERE
When does loss-of-exclusivity occur for BEVESPI AEROSPHERE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6621
Patent: COMPOSICIONES PARA EL SUMINISTRO DE ANTAGONISTAS MUSCARINICOS DE ACCION PROLONGADA Y AGONISTA DEL RECEPTOR ADRENERGICO B2 DE ACCION PROLONGADA. METODO DE TRATAMIENTO. SISTEMAS ASOCIADOS. INHALADOR DOSIFICADOR
Estimated Expiration: ⤷ Get Started Free
Patent: 6806
Patent: COMPOSICIONES PARA EL SUMINISTRO DE AGENTES ACTIVOS MEDIANTE LAS VIAS RES-PIRATORIAS, Y METODOS Y SISTEMAS ASOCIADOS, COSUSPENSION, INHALADOR DOSIFI-CADOR
Estimated Expiration: ⤷ Get Started Free
Patent: 6807
Patent: COMPOSICIONES METODOS Y SISTEMAS PARA EL SUMINISTRO DE DOS O MAS AGENTES ACTIVOS MEDIANTE LAS VIAS RESPIRATORIAS. METODO DE PREPARACION COMPOSICION
Estimated Expiration: ⤷ Get Started Free
Patent: 1758
Patent: COMPOSICIÓN FARMACÉUTICA QUE PUEDE SUMINISTRARSE DESDE UN INHALADOR DOSIFICADOR
Estimated Expiration: ⤷ Get Started Free
Patent: 2477
Patent: COMPOSICIONES PARA EL SUMINISTRO DE AGENTES ACTIVOS MEDIANTE LAS VÍAS RESPIRATORIAS, Y MÉTODOS Y SISTEMAS ASOCIADOS
Estimated Expiration: ⤷ Get Started Free
Patent: 2478
Patent: COMPOSICIONES PARA EL SUMINISTRO PULMONAR DE ANTAGONISTAS MUSCARÍNICOS DE ACCIÓN PROLONGADA Y AGONISTAS DEL RECEPTOR ADRENÉRGICO b₂ DE ACCIÓN PROLONGADA, Y MÉTODOS Y SISTEMAS ASOCIADOS
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10253770
Patent: Compositions for respiratory delivery of active agents and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 10253776
Patent: Compositions for pulmonary delivery of long-acting muscarinic antagonists and long-acting B2 adrenergic receptor agonists and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 10253950
Patent: Respiratory delivery of active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 15201037
Patent: Respiratory delivery of active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 17201709
Patent: Compositions, methods & systems for respiratory delivery of two or more active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 18282272
Patent: COMPOSITIONS, METHODS & SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS
Estimated Expiration: ⤷ Get Started Free
Patent: 20210160
Patent: COMPOSITIONS, METHODS & SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1011220
Patent: composição farmacêutica, métodos para tratar uma doença ou distúrbio, para administração respiratória de dois ou mais agentes ativos, para administração respiratória de uma combinação de agentes ativos, e, para preparar uma composição adequada.
Estimated Expiration: ⤷ Get Started Free
Patent: 1011229
Patent: co-suspensão, inalador de dose medida, e, métodos de preparação de um inalador de dose medida, de dispensação respiratória de um agente ativo a um paciente, e para tratar de um paciente.
Estimated Expiration: ⤷ Get Started Free
Patent: 1011508
Patent: composição farmacêutica, métodos para tratar uma doença ou distúrbio pulmonar em um paciente ou população de pacientes, e para dispensação respiratória de um agente ativo a um paciente, e, inalador de dose medida.
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 63936
Patent: COMPOSITIONS PERMETTANT L'ADMINISTRATION DE PRINCIPES ACTIFS PAR VOIE RESPIRATOIRE ET METHODES ET SYSTEMES ASSOCIES (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 63939
Patent: COMPOSITIONS PERMETTANT L'ADMINISTRATION PAR VOIE PULMONAIRE D'ANTAGONISTES, A ACTION PROLONGEE, DES RECEPTEURS MUSCARINIQUES ET D'AGONISTES, A ACTION PROLONGEE, DES RECEPTEURS ADRENERGIQUES B2 ET METHODES ET SYSTEMES ASSOCIES (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 63941
Patent: COMPOSITIONS, METHODES ET SYSTEMES PERMETTANT UNE ADMINISTRATION PAR VOIE RESPIRATOIRE DE DEUX PRINCIPES ACTIFS OU PLUS (COMPOSITIONS, METHODS & SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2458364
Patent: Compositions for pulmonary delivery of long-acting muscarinic antagonists and long-acting b2 adrenergic receptor agonists and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 2596176
Patent: Compositions for respiratory delivery of active agents and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 2753152
Patent: Compositions, methods & systems for respiratory delivery of two or more active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 5193773
Patent: Respiratory delivery of active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 7412212
Patent: 经肺递送长效毒蕈碱拮抗剂及长效β2肾上腺素能受体激动剂的组合物及相关方法与系统 (Compositions for pulmonary delivery of long-acting muscarinic antagonists and long-acting beta2 adrenergic receptor agonists and associated methods and systems)
Estimated Expiration: ⤷ Get Started Free
Patent: 7669664
Patent: 经呼吸递送活性剂的组合物及相关方法和系统 (Compositions For Respiratory Delivery Of Active Agents And Associated Methods And Systems)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0161098
Estimated Expiration: ⤷ Get Started Free
Patent: 0161101
Estimated Expiration: ⤷ Get Started Free
Patent: 0161102
Estimated Expiration: ⤷ Get Started Free
Patent: 0200166
Estimated Expiration: ⤷ Get Started Free
Patent: 0200260
Estimated Expiration: ⤷ Get Started Free
Patent: 0200298
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18030
Estimated Expiration: ⤷ Get Started Free
Patent: 18034
Estimated Expiration: ⤷ Get Started Free
Patent: 18040
Estimated Expiration: ⤷ Get Started Free
Patent: 22732
Estimated Expiration: ⤷ Get Started Free
Patent: 22749
Estimated Expiration: ⤷ Get Started Free
Patent: 22807
Estimated Expiration: ⤷ Get Started Free
Patent: 19031
Estimated Expiration: ⤷ Get Started Free
Patent: 21012
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 35023
Estimated Expiration: ⤷ Get Started Free
Patent: 35024
Estimated Expiration: ⤷ Get Started Free
Patent: 35025
Estimated Expiration: ⤷ Get Started Free
Patent: 06149
Estimated Expiration: ⤷ Get Started Free
Patent: 11926
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 35023
Patent: COMPOSITIONS PERMETTANT L'ADMINISTRATION PAR VOIE PULMONAIRE D'ANTAGONISTES, À ACTION PROLONGÉE, DES RÉCEPTEURS MUSCARINIQUES ET D'AGONISTES, À ACTION PROLONGÉE, DES RÉCEPTEURS ADRÉNERGIQUES 2 ET MÉTHODES ET SYSTÈMES ASSOCIÉS (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING BETA 2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 35024
Patent: COMPOSITIONS PERMETTANT L'ADMINISTRATION DE PRINCIPES ACTIFS PAR VOIE RESPIRATOIRE ET MÉTHODES ET SYSTÈMES ASSOCIÉS (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 35025
Patent: ADMINISTRATION PAR VOIE RESPIRATOIRE DE PRINCIPES ACTIFS (RESPIRATORY DELIVERY OF ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 06149
Patent: COMPOSITIONS POUR L'ADMINISTRATION PAR VOIE PULMONAIRE D'ANTAGONISTES MUSCARINIQUES ET AGONISTES DU RÉCEPTEUR ADRÉNERGIQUE BÉTA-2 À ACTION PROLONGÉE, PROCÉDÉS ET SYSTÈMES ASSOCIÉS (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING BETA-2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 11926
Patent: COMPOSITIONS, PROCÉDÉS ET SYSTÈMES POUR UNE ADMINISTRATION RESPIRATOIRE DE DEUX OU DE PLUSIEURS AGENTS ACTIFS (COMPOSITIONS, METHODS & SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Patent: COMPOSITIONS POUR ADMINISTRATION RESPIRATOIRE D'AGENTS ACTIFS ET MÉTHODES ET SYSTÈMES ASSOCIÉS (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1040
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 69026
Patent: 用於經由肺部遞送長效蕈毒鹼拮抗劑及長效β 腎上腺素受體激動劑之組成物,及相關方法及系統 (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING BETA ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 69027
Patent: 用於經由呼吸道輸送活性劑的組成物以及相關方法與系統 (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 69307
Patent: 經由呼吸道遞送活性藥劑 (RESPIRATORY DELIVERY OF ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 18867
Patent: 活性劑的呼吸遞送 (RESPIRATORY DELIVERY OF ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 44669
Patent: 經肺遞送長效毒蕈堿拮抗劑及長效β2腎上腺素能受體激動劑的組合物及相關方法與系統 (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING β2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 47095
Patent: 經呼吸遞送活性劑的組合物及相關方法和系統 (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 29532
Estimated Expiration: ⤷ Get Started Free
Patent: 31229
Estimated Expiration: ⤷ Get Started Free
Patent: 31283
Estimated Expiration: ⤷ Get Started Free
Patent: 47803
Estimated Expiration: ⤷ Get Started Free
Patent: 47823
Estimated Expiration: ⤷ Get Started Free
Patent: 47834
Estimated Expiration: ⤷ Get Started Free
Patent: 900031
Estimated Expiration: ⤷ Get Started Free
Patent: 100018
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6466
Patent: תכשירים לנתינה למערכת הנשימה של גורמים פעילים ושיטות ומערכות קשורות (Compositions for respiratory delivery of active agents and associated methods and systems)
Estimated Expiration: ⤷ Get Started Free
Patent: 6467
Patent: תרכובות פרמצבטיות למתן באמצעות משאף מינוני המכילות גליקופירולט או פורמוטרול ושמושן לטיפול במחלות נשימתיות (Pharmaceutical compositions deliverable from a metered dose inhaler comprising glycopyrrolate or formoterol and their use in treatment of pulmonary diseases)
Estimated Expiration: ⤷ Get Started Free
Patent: 6468
Patent: תרכובות פרמצבטיות למתן באמצעות משאף מינוני המכילות גליקופירולט ופורמוטרול ושמושן לטיפול במחלות נשימתיות (Pharmaceutical compositions deliverable from a metered dose inhaler comprising glycopyrrolate and formoterol and their use in treatment of pulmonary diseases)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 23383
Estimated Expiration: ⤷ Get Started Free
Patent: 73012
Estimated Expiration: ⤷ Get Started Free
Patent: 73013
Estimated Expiration: ⤷ Get Started Free
Patent: 69639
Estimated Expiration: ⤷ Get Started Free
Patent: 89356
Estimated Expiration: ⤷ Get Started Free
Patent: 48645
Estimated Expiration: ⤷ Get Started Free
Patent: 92124
Estimated Expiration: ⤷ Get Started Free
Patent: 76734
Estimated Expiration: ⤷ Get Started Free
Patent: 12528199
Estimated Expiration: ⤷ Get Started Free
Patent: 12528200
Estimated Expiration: ⤷ Get Started Free
Patent: 12528792
Estimated Expiration: ⤷ Get Started Free
Patent: 15187108
Patent: 活性剤を呼吸器送達するための組成物、ならびに関連する方法および系 (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 15199735
Patent: 2つ以上の活性剤を呼吸器送達するための組成物、方法および系 (COMPOSITION, METHOD AND SYSTEM FOR DELIVERING TWO OR MORE ACTIVATORS TO RESPIRATORY ORGAN)
Estimated Expiration: ⤷ Get Started Free
Patent: 16041713
Patent: 長時間作用性のムスカリン拮抗剤および長時間作用性のΒ2アドレナリン受容体作動剤を肺送達するための組成物ならびに関連の方法および系 (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING BETA2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 17222706
Patent: 2つ以上の活性剤を呼吸器送達するための組成物、方法および系 (COMPOSITIONS, METHODS AND SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 18008942
Patent: 活性剤を呼吸器送達するための組成物、ならびに関連する方法および系 (COMPOSITION FOR RESPIRATORY DELIVERY OF ACTIVE AGENT, AND RELATED METHOD AND SYSTEM)
Estimated Expiration: ⤷ Get Started Free
Patent: 18048150
Patent: 長時間作用性のムスカリン拮抗剤および長時間作用性のΒ2アドレナリン受容体作動剤を肺送達するための組成物ならびに関連の方法および系 (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS, AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19108369
Patent: 活性剤を呼吸器送達するための組成物、ならびに関連する方法および系 (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 435024
Estimated Expiration: ⤷ Get Started Free
Patent: 435025
Estimated Expiration: ⤷ Get Started Free
Patent: 2019014
Estimated Expiration: ⤷ Get Started Free
Patent: 2021511
Estimated Expiration: ⤷ Get Started Free
Patent: 35023
Estimated Expiration: ⤷ Get Started Free
Patent: 35024
Estimated Expiration: ⤷ Get Started Free
Patent: 35025
Estimated Expiration: ⤷ Get Started Free
Patent: 06149
Estimated Expiration: ⤷ Get Started Free
Patent: 11926
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0124
Estimated Expiration: ⤷ Get Started Free
Patent: 0208
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 7126
Patent: ADMINISTRACION RESPIRATORIA DE AGENTES ACTIVOS. (RESPIRATORY DELIVERY OF ACTIVE AGENTS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 0163
Patent: COMPOSICIONES PARA SUMINISTRO RESPIRATORIO DE AGENTES ACTIVOS Y METODOS Y SISTEMAS ASOCIADOS. (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 0164
Patent: COMPOSICIONES PARA SUMINISTRO PULMONAR DE ANTAGONISTAS MUSCARÍNICOS DE ACCIÓN PROLONGADA Y AGONISTAS DE RECEPTOR B2 ADRENÉRGICO DE ACCIÓN PROLONGADA Y MÉTODOS Y SISTEMAS ASOCIADOS. (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 7778
Patent: COMPOSICIONES DE MICROPARTÍCULAS DE GLICOPIRROLATO ADAPTADAS PARA EL SUMINISTRO RESPIRATORIO MEDIANTE UN INHALADOR DE DOSIS MEDIDA Y EL USO DE LAS MISMAS PARA EL TRATAMIENTO DE TRASTORNOS PULMONARES. (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 3243
Patent: COMPOSICIÓN FARMACÉUTICA QUE COMPRENDE LA COMBINACIÓN DE ALBUTEROL Y BUDESÓNIDA, ADAPTADA PARA EL SUMINISTRO RESPIRATORIO MEDIANTE UN INHALADOR DE DOSIS MEDIDA Y EL USO DE LAS MISMAS PARA EL TRATAMIENTO DE TRASTORNOS PULMONARES. (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 11012684
Patent: COMPOSICIONES PARA SUMINISTRO RESPIRATORIO DE AGENTES ACTIVOS Y METODOS Y SISTEMAS ASOCIADOS. (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11012685
Patent: COMPOSICIONES PARA SUMINISTRO PULMONAR DE ANTAGONISTAS MUSCARINICOS DE ACCION PROLONGADA Y AGONISTAS DE RECEPTOR B2 ADRENERGICO DE ACCION PROLONGADA Y METODOS Y SISTEMAS ASOCIADOS. (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11012783
Patent: ADMINISTRACION RESPIRATORIA DE AGENTES ACTIVOS. (RESPIRATORY DELIVERY OF ACTIVE AGENTS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 20004077
Patent: COMPOSICIÓN FARMACÉUTICA QUE COMPRENDE LA COMBINACIÓN DE ALBUTEROL Y BUDESÓNIDA, ADAPTADA PARA EL SUMINISTRO RESPIRATORIO MEDIANTE UN INHALADOR DE DOSIS MEDIDA Y EL USO DE LAS MISMAS PARA EL TRATAMIENTO DE TRASTORNOS PULMONARES. (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 631
Patent: SASTAVI ZA ISPORUKU AKTIVNIH AGENASA U DISAJNE PUTEVE I POVEZANI POSTUPCI I SISTEMI (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 19026
Estimated Expiration: ⤷ Get Started Free
Patent: 21019
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 017500778
Patent: COMPOSITIONS, METHODS and SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 35023
Estimated Expiration: ⤷ Get Started Free
Patent: 35024
Estimated Expiration: ⤷ Get Started Free
Patent: 35025
Estimated Expiration: ⤷ Get Started Free
Patent: 06149
Estimated Expiration: ⤷ Get Started Free
Patent: 11926
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 35023
Estimated Expiration: ⤷ Get Started Free
Patent: 35024
Estimated Expiration: ⤷ Get Started Free
Patent: 35025
Estimated Expiration: ⤷ Get Started Free
Patent: 06149
Estimated Expiration: ⤷ Get Started Free
Patent: 11926
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 80315
Patent: КОМПОЗИЦИИ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ АКТИВНЫХ ВЕЩЕСТВ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE SUBSTANCES AND METHODS AND SYSTEMS CONNECTED THEREWITH)
Estimated Expiration: ⤷ Get Started Free
Patent: 86297
Patent: КОМПОЗИЦИИ, СПОСОБЫ И СИСТЕМЫ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ ДВУХ ИЛИ БОЛЕЕ АКТИВНЫХ АГЕНТОВ (COMPOSITIONS, METHODS AND SYSTEMS FOR RESPIRATORY DELIVERY OF TWO OR MORE ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 13404
Patent: КОМПОЗИЦИИ ДЛЯ ЛЕГОЧНОЙ ДОСТАВКИ АНТАГОНИСТОВ МУСКАРИНОВЫХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И АГОНИСТОВ В2-АДРЕНЕРГИЧЕСКИХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC RECEPTOR ANTAGONISTS AND LONG-ACTING B2-ADRENERGIC RECEPTOR AGONISTS AND METHODS AND SYSTEMS ASSOCIATED THEREWITH)
Estimated Expiration: ⤷ Get Started Free
Patent: 51771
Patent: КОМПОЗИЦИИ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ АКТИВНЫХ ВЕЩЕСТВ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE SUBSTANCES AND METHODS AND SYSTEMS ASSOCIATED THEREWITH)
Estimated Expiration: ⤷ Get Started Free
Patent: 11152960
Patent: КОМПОЗИЦИИ ДЛЯ ЛЕГОЧНОЙ ДОСТАВКИ АНТАГОНИСТОВ МУСКАРИНОВЫХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И АГОНИСТОВ β-АДРЕНЕРГИЧЕСКИХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ
Estimated Expiration: ⤷ Get Started Free
Patent: 11154083
Patent: КОМПОЗИЦИИ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ АКТИВНЫХ ВЕЩЕСТВ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ
Estimated Expiration: ⤷ Get Started Free
Patent: 11154148
Patent: КОМПОЗИЦИИ, СПОСОБЫ И СИСТЕМЫ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ ДВУХ ИЛИ БОЛЕЕ АКТИВНЫХ АГЕНТОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 15151358
Patent: КОМПОЗИЦИИ ДЛЯ ЛЕГОЧНОЙ ДОСТАВКИ АНТАГОНИСТОВ МУСКАРИНОВЫХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И АГОНИСТОВ В2-АДРЕНЕРГИЧЕСКИХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ
Estimated Expiration: ⤷ Get Started Free
Patent: 16107464
Patent: КОМПОЗИЦИИ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ АКТИВНЫХ ВЕЩЕСТВ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ
Estimated Expiration: ⤷ Get Started Free
Patent: 16117972
Patent: КОМПОЗИЦИИ, СПОСОБЫ И СИСТЕМЫ ДЛЯ РЕСПИРАТОРНОЙ ДОСТАВКИ ДВУХ ИЛИ БОЛЕЕ АКТИВНЫХ АГЕНТОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 20102859
Patent: КОМПОЗИЦИИ ДЛЯ ЛЕГОЧНОЙ ДОСТАВКИ АНТАГОНИСТОВ МУСКАРИНОВЫХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И АГОНИСТОВ B2-АДРЕНЕРГИЧЕСКИХ РЕЦЕПТОРОВ ДЛИТЕЛЬНОГО ДЕЙСТВИЯ И СВЯЗАННЫЕ С НИМИ СПОСОБЫ И СИСТЕМЫ
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01600326
Patent: DISTRIBUZIONE RESPIRATORIA DI PRINCIPI ATTIVI
Estimated Expiration: ⤷ Get Started Free
Patent: 01600327
Patent: COMPOSIZIONI PER IL RILASCIO POLMONARE DI ANTAGONISTI MUSCARINICI AD AZIONE PROLUNGATA E DI AGONISTI DEL RECETTORE ADRENERGICO BETA 2 AD AZIONE PROLUNGATA E METODI E SISTEMI ASSOCIATI
Estimated Expiration: ⤷ Get Started Free
Patent: 01600329
Patent: COMPOSIZIONI PER LA DISTRIBUZIONE RESPIRATORIA DI PRINCIPI ATTIVI E METODI E SISTEMI
ASSOCIATI
Estimated Expiration: ⤷ Get Started Free
Patent: 02000077
Estimated Expiration: ⤷ Get Started Free
Patent: 02000108
Estimated Expiration: ⤷ Get Started Free
Patent: 02000109
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 35023
Estimated Expiration: ⤷ Get Started Free
Patent: 35024
Estimated Expiration: ⤷ Get Started Free
Patent: 35025
Estimated Expiration: ⤷ Get Started Free
Patent: 06149
Estimated Expiration: ⤷ Get Started Free
Patent: 11926
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1108275
Patent: RESPIRATORY DELIVERY OF ACTIVE AGENTS
Estimated Expiration: ⤷ Get Started Free
Patent: 1208100
Patent: COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS
Estimated Expiration: ⤷ Get Started Free
Patent: 1208101
Patent: COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS
Estimated Expiration: ⤷ Get Started Free
Patent: 1208102
Patent: RESPIRATORY DELIVERY OF ACTIVE AGENTS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1748892
Estimated Expiration: ⤷ Get Started Free
Patent: 1926060
Estimated Expiration: ⤷ Get Started Free
Patent: 1976107
Estimated Expiration: ⤷ Get Started Free
Patent: 120015334
Patent: COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS
Estimated Expiration: ⤷ Get Started Free
Patent: 120026075
Patent: RESPIRATORY DELIVERY OF ACTIVE AGENTS
Estimated Expiration: ⤷ Get Started Free
Patent: 120034631
Patent: COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS
Estimated Expiration: ⤷ Get Started Free
Patent: 170070274
Patent: 지속형 무스카린 안타고니스트 및 지속형 B₂아드레날린 수용체 아고니스트의 폐 전달용 조성물, 및 연관된 방법 및 시스템 (COMPOSITIONS FOR PULMONARY DELIVERY OF LONG-ACTING MUSCARINIC ANTAGONISTS AND LONG-ACTING B2 ADRENERGIC RECEPTOR AGONISTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 170104003
Patent: 활성제의 호흡기 전달용 조성물, 및 연관된 방법 및 시스템 (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 180130602
Patent: 활성제의 호흡기 전달용 조성물, 및 연관된 방법 및 시스템 (COMPOSITIONS FOR RESPIRATORY DELIVERY OF ACTIVE AGENTS AND ASSOCIATED METHODS AND SYSTEMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 190049943
Patent: 활성제의 호흡기 전달 (RESPIRATORY DELIVERY OF ACTIVE AGENTS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 89135
Estimated Expiration: ⤷ Get Started Free
Patent: 92536
Estimated Expiration: ⤷ Get Started Free
Patent: 93429
Estimated Expiration: ⤷ Get Started Free
Patent: 72253
Estimated Expiration: ⤷ Get Started Free
Patent: 74367
Estimated Expiration: ⤷ Get Started Free
Patent: 74391
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 39979
Estimated Expiration: ⤷ Get Started Free
Patent: 46094
Estimated Expiration: ⤷ Get Started Free
Patent: 32926
Estimated Expiration: ⤷ Get Started Free
Patent: 33898
Estimated Expiration: ⤷ Get Started Free
Patent: 46980
Estimated Expiration: ⤷ Get Started Free
Patent: 95723
Estimated Expiration: ⤷ Get Started Free
Patent: 07700
Estimated Expiration: ⤷ Get Started Free
Patent: 17511
Estimated Expiration: ⤷ Get Started Free
Patent: 92140
Estimated Expiration: ⤷ Get Started Free
Patent: 1109049
Patent: Compositions for respiratory delivery of active agents and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 1109050
Patent: Compositions for pulmonary delivery of long-acting muscarinic antagonists and long-acting &bgr;2 adrenergic receptor agonists and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 1109051
Patent: Compositions, methods and systems for respiratory delivery of two or more active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 1642836
Patent: Compositions for pulmonary delivery of long-acting muscarinic antagonists and long-acting [beta]2 adrenergic receptor agonists and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 1700123
Patent: Compositions for respiratory delivery of active agents and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 1808372
Patent: Compositions, methods and systems for respiratory delivery of two or more active agents
Estimated Expiration: ⤷ Get Started Free
Patent: 1919730
Patent: Compositions for respiratory delivery of active agents and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 1936174
Patent: Compositions for pulmonary delivery of long-acting muscarinic antagonists and long-acting [beta]2 adrenergic receptor agonists and associated methods and systems
Estimated Expiration: ⤷ Get Started Free
Patent: 2114642
Patent: Compositions, methods and systems for respiratory delivery of two or more active agents
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 9529
Patent: КОМПОЗИЦІЇ ДЛЯ РЕСПІРАТОРНОЇ ДОСТАВКИ АКТИВНИХ АГЕНТІВ ТА ПОВ'ЯЗАНІ СПОСОБИ І СИСТЕМИ
Estimated Expiration: ⤷ Get Started Free
Patent: 9530
Patent: КОМПОЗИЦІЇ, СПОСОБИ ТА СИСТЕМИ ДЛЯ ДОСТАВКИ РЕСПІРАТОРНИМ ШЛЯХОМ ДВОХ ЧИ БІЛЬШЕ АКТИВНИХ АГЕНТІВ
Estimated Expiration: ⤷ Get Started Free
Patent: 9531
Patent: КОМПОЗИЦІЇ ДЛЯ ЛЕГЕНЕВОЇ ДОСТАВКИ МУСКАРИНОВИХ АНТАГОНІСТІВ ТРИВАЛОЇ ДІЇ ТА АГОНІСТІВ АДРЕНЕРГІЧНИХ РЕЦЕПТОРІВ B2 ТРИВАЛОЇ ДІЇ ТА ПОВ'ЯЗАНІ СПОСОБИ І СИСТЕМИ
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BEVESPI AEROSPHERE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2018008942 | 活性剤を呼吸器送達するための組成物、ならびに関連する方法および系 (COMPOSITION FOR RESPIRATORY DELIVERY OF ACTIVE AGENT, AND RELATED METHOD AND SYSTEM) | ⤷ Get Started Free |
| Japan | 2012528792 | ⤷ Get Started Free | |
| Brazil | PI1011220 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BEVESPI AEROSPHERE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435025 | 2019026 | Norway | ⤷ Get Started Free | PRODUCT NAME: KOMBINASJON AV GLYKOPYRROLAT (INKLUDERT EVENTUELLE FARMASOEYTISK AKSEPTABLE SALTER, ESTERE ELLER ENANTIOMERER DERAV) OG FORMOTEROL (INNBEFATTENDE HVILKE SOM HELST FARMASOEYTISK AKSEPTABLE SALTER, ESTERE ELLER ENANTIOMERER DERAV); REG. NO/DATE: EU/1/18/1339 20190104 |
| 2435025 | 122019000068 | Germany | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION VON GLYCOPYRROLAT, EINSCHLIESSLICH BELIEBIGER PHARMAZEUTISCH VERTRAEGLICHER SALZE, ESTER, ENANTIOMERE, ODER ANDERER DERIVATE DAVON, UND FORMOTEROL, EINSCHLIESSLICH BELIEBIGER PHARMAZEUTISCH VERTRAEGLICHER SALZE, ESTER, ENANTIOMERE, ODER ANDERER DERIVATE DAVON; REGISTRATION NO/DATE: EU/1/18/1339 20181218 |
| 2435025 | 132019000000087 | Italy | ⤷ Get Started Free | PRODUCT NAME: UNA COMBINAZIONE DI GLICOPIRROLATO (INCLUSI SUOI SALI, ESTERI, ENANTIOMERI O ALTRI DERIVATI FARMACEUTICAMENTE ACCETTABILI) E FORMOTEROLO (INCLUSI SUOI SALI, ESTERI, ENANTIOMERI O ALTRI DERIVATI FARMACEUTICAMENTE ACCETTABILI)(BEVESPI AEROSPHERE); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1339, 20181220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for BEVESPI AEROSPHERE
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
