ATRIPLA Drug Patent Profile
✉ Email this page to a colleague
When do Atripla patents expire, and what generic alternatives are available?
Atripla is a drug marketed by Gilead Sciences and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and twenty-three patent family members in thirty-one countries.
The generic ingredient in ATRIPLA is efavirenz; emtricitabine; tenofovir disoproxil fumarate. There are twenty-six drug master file entries for this compound. Eight suppliers are listed for this compound. Additional details are available on the efavirenz; emtricitabine; tenofovir disoproxil fumarate profile page.
DrugPatentWatch® Generic Entry Outlook for Atripla
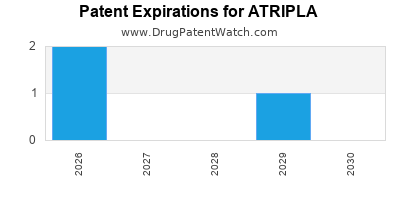
Atripla was eligible for patent challenges on July 2, 2007.
There have been eighteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (efavirenz; emtricitabine; tenofovir disoproxil fumarate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for ATRIPLA
| International Patents: | 123 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 54 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ATRIPLA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ATRIPLA |
| What excipients (inactive ingredients) are in ATRIPLA? | ATRIPLA excipients list |
| DailyMed Link: | ATRIPLA at DailyMed |

Recent Clinical Trials for ATRIPLA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Willem Daniel Francois Venter | Phase 1 |
| University of Cape Town | Phase 1 |
| Yu-Jay Corp. | Phase 3 |
Pharmacology for ATRIPLA
Anatomical Therapeutic Chemical (ATC) Classes for ATRIPLA
Paragraph IV (Patent) Challenges for ATRIPLA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ATRIPLA | Tablets | efavirenz; emtricitabine; tenofovir disoproxil fumarate | 600 mg/200 mg/300 mg | 021937 | 1 | 2008-12-29 |
US Patents and Regulatory Information for ATRIPLA
ATRIPLA is protected by ten US patents.
Patents protecting ATRIPLA
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN ADULTS
Unitary pharmaceutical dosage form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV INFECTION
Unitary pharmaceutical dosage form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN ADULTS
Unitary pharmaceutical dosage form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV INFECTION
Unitary pharmaceutical dosage form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
Unitary pharmaceutical dosage form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN ADULTS
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV INFECTION
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ATRIPLA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead Sciences | ATRIPLA | efavirenz; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021937-001 | Jul 12, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ATRIPLA
See the table below for patents covering ATRIPLA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2017057233 | ヌクレオチドアナログ組成物および合成方法 (NUCLEOTIDE ANALOG COMPOSITION AND SYNTHESIS METHOD) | ⤷ Try a Trial |
| Portugal | 1067936 | ⤷ Try a Trial | |
| New Zealand | 336510 | Process for the crystallization of a reverse transcriptase inhibitor using an anti-solvent | ⤷ Try a Trial |
| New Zealand | 540728 | Compositions and methods for combination antiviral therapy | ⤷ Try a Trial |
| Japan | 5557999 | ⤷ Try a Trial | |
| China | 1920050 | ⤷ Try a Trial | |
| Japan | 3987335 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ATRIPLA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0513200 | 7/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: EMTRICITABIN; NAT. REGISTRATION NO/DATE: EU/1/03/261/001- EU/1/03/261/003 20031024; FIRST REGISTRATION: EU EU/1/03/261/003 |
| 0915894 | CA 2008 00023 | Denmark | ⤷ Try a Trial | |
| 0915894 | 08C0020 | France | ⤷ Try a Trial | PRODUCT NAME: EFAVIRENZ; EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE; REGISTRATION NO/DATE: EU/1/07/430/001 20071213 |
| 0582455 | SPC029/2000 | Ireland | ⤷ Try a Trial | SPC029/2000: 20050912, EXPIRES: 20131119 |
| 0915894 | C00915894/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: TENOFOVIR DISOPROXILFUMARAT + EMTRICITABIN; REGISTRATION NUMBER/DATE: SWISSMEDIC 57316 21.03.2006 |
| 0582455 | 2008/016 | Ireland | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF EFAVIRENZ AND EMTRICTABINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTRATION NO/DATE: EU/1/07/430/001 20071213 |
| 0582455 | SPC/GB00/035 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: EFAVIRENZ, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: CH IKS-54 908 01 19981120; CH IKS-54 908 02 19981120; CH IKS-54 908 03 19981120; UK EU/1/99/110/001 19990528; UK EU/1/99/110/002 19990528; UK EU/1/99/110/003 19990528; UK EU/1/99/110/004 19990528; UK EU/1/99/111/001 19990528; UK EU/1/99/111/002 19990528; UK EU/1/99/111/003 19990528; UK EU/1/99/111/004 19990528 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


