ARAZLO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Arazlo, and when can generic versions of Arazlo launch?
Arazlo is a drug marketed by Bausch and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has twenty-seven patent family members in nineteen countries.
The generic ingredient in ARAZLO is tazarotene. There are eight drug master file entries for this compound. Eleven suppliers are listed for this compound. Additional details are available on the tazarotene profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Arazlo
A generic version of ARAZLO was approved as tazarotene by TARO on April 3rd, 2017.
Summary for ARAZLO
| International Patents: | 27 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 129 |
| Clinical Trials: | 1 |
| Patent Applications: | 4,408 |
| Formulation / Manufacturing: | see details |
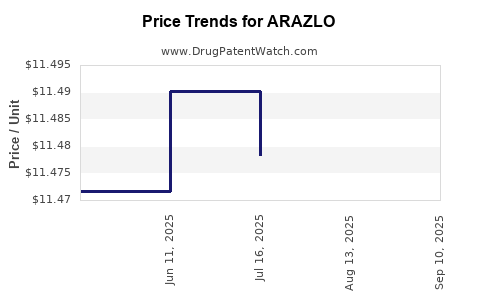
| Drug Prices: | Drug price information for ARAZLO |
| What excipients (inactive ingredients) are in ARAZLO? | ARAZLO excipients list |
| DailyMed Link: | ARAZLO at DailyMed |
Recent Clinical Trials for ARAZLO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dr. Emmy Graber | Phase 4 |
Pharmacology for ARAZLO
| Drug Class | Retinoid |
Anatomical Therapeutic Chemical (ATC) Classes for ARAZLO
Paragraph IV (Patent) Challenges for ARAZLO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ARAZLO | Topical Lotion | tazarotene | 0.045% | 211882 | 1 | 2022-05-12 |
US Patents and Regulatory Information for ARAZLO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch | ARAZLO | tazarotene | LOTION;TOPICAL | 211882-001 | Dec 18, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bausch | ARAZLO | tazarotene | LOTION;TOPICAL | 211882-001 | Dec 18, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ARAZLO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | ARAZLO | tazarotene | LOTION;TOPICAL | 211882-001 | Dec 18, 2019 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ARAZLO
See the table below for patents covering ARAZLO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2251410 | СИСТЕМА ДОСТАВКИ В ВИДЕ ГЕЛЯ ДЛЯ МЕСТНОГО ПРИМЕНЕНИЯ (DELIVERY SYSTEM IN THE FORM OF GEL FOR LOCAL APPLICATION) | ⤷ Sign Up |
| South Korea | 102643849 | ⤷ Sign Up | |
| Slovenia | 1304992 | ⤷ Sign Up | |
| World Intellectual Property Organization (WIPO) | 0211683 | ⤷ Sign Up | |
| Denmark | 1304992 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ARAZLO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1304992 | 92401 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: CLINDAMYCINE(EN TANT QUE PHOPSHATE DE CLINDAMYCINE)ET TRETINOINE |
| 0284288 | 12/1998 | Austria | ⤷ Sign Up | PRODUCT NAME: TAZAROTENE; NAT. REGISTRATION NO/DATE: 1-22102, 1-22103 19970918; FIRST REGISTRATION: DE 37393.00.00, 37393.01.00 19961203 |
| 0031058 | 98C0008 | Belgium | ⤷ Sign Up | PRODUCT NAME: TAZAROTENE; NAT. REGISTRATION NO/DATE: NL22604 19970922; FIRST REGISTRATION: DE - 37393.00.00 19961203 |
| 1304992 | 132013902214376 | Italy | ⤷ Sign Up | PRODUCT NAME: CLINDAMICINA FOSFATO E TRETINOINA(ACNATAC); AUTHORISATION NUMBER(S) AND DATE(S): PA1332/043/001, 20130322;042056010/M - 022/M, 20130718 |
| 1304992 | 1390049-3 | Sweden | ⤷ Sign Up | PRODUCT NAME: KLINDAMYCIN (SOM KLINDAMYCINFOSFAT) OCH TRETINOIN; NAT. REG. NO/DATE: MTNR 46193 20130503; FIRST REG.: IR PA1332/043/001 20130322 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


