ANORO ELLIPTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Anoro Ellipta, and when can generic versions of Anoro Ellipta launch?
Anoro Ellipta is a drug marketed by Glaxosmithkline and is included in one NDA. There are twelve patents protecting this drug.
This drug has three hundred and twenty-nine patent family members in forty-eight countries.
The generic ingredient in ANORO ELLIPTA is umeclidinium bromide; vilanterol trifenatate. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the umeclidinium bromide; vilanterol trifenatate profile page.
DrugPatentWatch® Generic Entry Outlook for Anoro Ellipta
Anoro Ellipta was eligible for patent challenges on May 10, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 29, 2030. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for ANORO ELLIPTA?
- What are the global sales for ANORO ELLIPTA?
- What is Average Wholesale Price for ANORO ELLIPTA?
Summary for ANORO ELLIPTA
| International Patents: | 329 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 57 |
| Clinical Trials: | 47 |
| Patent Applications: | 289 |
| Drug Prices: | Drug price information for ANORO ELLIPTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ANORO ELLIPTA |
| What excipients (inactive ingredients) are in ANORO ELLIPTA? | ANORO ELLIPTA excipients list |
| DailyMed Link: | ANORO ELLIPTA at DailyMed |
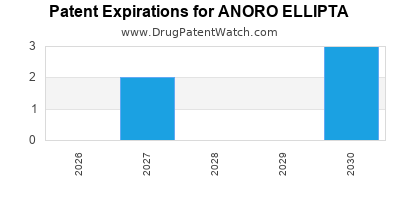
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ANORO ELLIPTA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ANORO ELLIPTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Baylor Research Institute | Phase 4 |
| Chiesi Farmaceutici S.p.A. | Phase 4 |
| Luis Puente Maestu | Phase 4 |
Pharmacology for ANORO ELLIPTA
| Drug Class | Anticholinergic beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists Cholinergic Antagonists |
US Patents and Regulatory Information for ANORO ELLIPTA
ANORO ELLIPTA is protected by twelve US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ANORO ELLIPTA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ANORO ELLIPTA
Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: MAINTENANCE TREATMENT OF PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
Phenethanolamine derivatives for treatment of respiratory diseases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Muscarinic acetylcholine receptor antagonists
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Muscarinic acetylcholine receptor antagonists
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Medicament dispenser
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Muscarinic acetylcholine receptor antagonists
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED FOR THE LONG-TERM, ONCE-DAILY, MAINTENANCE TREATMENT OF AIRFLOW OBSTRUCTION IN PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD), INCLUDING CHRONIC BRONCHITIS AND/OR EMPHYSEMA.
Muscarinic acetylcholine receptor antagonists
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED FOR THE LONG-TERM, ONCE-DAILY, MAINTENANCE TREATMENT OF AIRFLOW OBSTRUCTION IN PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD), INCLUDING CHRONIC BRONCHITIS AND/OR EMPHYSEMA.
Medicament dispenser
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Manifold for use in medicament dispenser
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Medicament dispenser
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Medicament dispenser
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Expired US Patents for ANORO ELLIPTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ANORO ELLIPTA
When does loss-of-exclusivity occur for ANORO ELLIPTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10326798
Patent: Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Estimated Expiration: ⤷ Sign Up
Patent: 14204459
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Patent: 16262698
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Patent: 17238392
Patent: Mannose-derived antagonists of FimH useful for treating disease
Estimated Expiration: ⤷ Sign Up
Patent: 18282427
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Patent: 21204302
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Patent: 23219901
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2012012925
Patent: produto de combinação farmacêutico, inalador de pó seco, e, uso de um produto
Estimated Expiration: ⤷ Sign Up
Patent: 2018069147
Patent: composto da fórmula iii, composição farmacêutica, método de inibição da função de fimh e método de tratamento de uma doença mediada por fimh
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 81487
Patent: COMBINAISONS D'UN ANTAGONISTE DE RECEPTEUR MUSCARINIQUE ET D'UN AGONISTE DU RECEPTEUR BETA-2 ADRENERGIQUE (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Patent: 18345
Patent: ANTAGONISTES DERIVES DU MANNOSE DE FIMH UTILES POUR LE TRAITEMENT D'UNE MALADIE (MANNOSE-DERIVED ANTAGONISTS OF FIMH USEFUL FOR TREATING DISEASE)
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 12001432
Patent: Producto de combinacion farmacéutica que comprende bromuro de 4-[hidroxi-(difenil)-metil]-1-{2-[(fenil-metil)-oxi]-etil}-1-azonia-biciclo-[2.2.2]-octano y trifenil-acetato de 4-{(1r)-2-[(6-{2-[(2,6-dicloro-bencil)-oxi]-etoxi}-hexil)-amino]-1-hidroxi-etil}-2-(hidroxi-metil)-fenol; y su uso para tratar enfermedades respiratorias.
Estimated Expiration: ⤷ Sign Up
China
Patent: 2724974
Patent: Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Estimated Expiration: ⤷ Sign Up
Patent: 7412229
Patent: 毒蕈碱受体拮抗剂和β‑2肾上腺素受体激动剂的组合 (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Patent: 8778288
Patent: 可用于治疗疾病的FimH的甘露糖衍生的拮抗剂 (Mannose-derived antagonists of FimH useful for treating disease)
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 41613
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA 2
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 120265
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0180312
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 20058
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 06844
Estimated Expiration: ⤷ Sign Up
Dominican Republic
Patent: 012000148
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 3839
Patent: КОМБИНАЦИИ АНТАГОНИСТА МУСКАРИНОВЫХ РЕЦЕПТОРОВ И АГОНИСТА БЕТА-2-АДРЕНОРЕЦЕПТОРОВ (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Patent: 1290266
Patent: КОМБИНАЦИИ АНТАГОНИСТА МУСКАРИНОВЫХ РЕЦЕПТОРОВ И АГОНИСТА БЕТА-2-АДРЕНОРЕЦЕПТОРОВ
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 06844
Patent: Combinaisons d'un antagoniste de récepteur muscarinique et d'un agoniste du récepteur bêta-2 adrénergique (Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist)
Estimated Expiration: ⤷ Sign Up
Patent: 35707
Patent: COMBINAISONS D'UN ANTAGONISTE DE RÉCEPTEUR MUSCARINIQUE ET D'UN AGONISTE DU RÉCEPTEUR BÊTA-2 ADRÉNERGIQUE (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Patent: 32892
Patent: ANTAGONISTES DÉRIVÉS DU MANNOSE DE FIMH UTILES POUR LE TRAITEMENT D'UNE MALADIE (MANNOSE-DERIVED ANTAGONISTS OF FIMH USEFUL FOR TREATING DISEASE)
Estimated Expiration: ⤷ Sign Up
France
Patent: C1022
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 49407
Patent: 毒蕈碱受體拮抗劑和β-2腎上腺素受體激動劑的組合 (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 36216
Estimated Expiration: ⤷ Sign Up
Patent: 800027
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 9893
Patent: שילובים של אנטגוניסט לקולטן מוסקריני ואגוניסט לקולטן בתא-2-אדרנו (Combinations of a muscarinic receptor antagonist and a beta-2 adrenorecptor agonist)
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 16631
Estimated Expiration: ⤷ Sign Up
Patent: 13512270
Estimated Expiration: ⤷ Sign Up
Patent: 19509315
Patent: 疾患の処置に有用なFimHのマンノース由来アンタゴニスト
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 506844
Estimated Expiration: ⤷ Sign Up
Patent: 2018011
Estimated Expiration: ⤷ Sign Up
Patent: 06844
Estimated Expiration: ⤷ Sign Up
Luxembourg
Patent: 0077
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 4864
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 1290
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2. (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST.)
Estimated Expiration: ⤷ Sign Up
Patent: 12006310
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2. (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST.)
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 965
Patent: KOMBINACIJE ANTAGONISTA MUSKARINSKOG RECEPTORA I AGONISTA ВЕТА - 2 ADRENORECEPTORA (Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist)
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 853
Patent: تركيبة مضاد استقبال المسكارينية ومحضر استقبال بتي ـ 2 الأدرينالية
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 0026
Patent: Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 06844
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 130042
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Sign Up
Patent: 170915
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 06844
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 06844
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 18131440
Patent: Антагонисты FimH, являющиеся производными маннозы, пригодные для лечения заболевания
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 848
Patent: KOMBINACIJE ANTAGONISTA MUSKARINSKOG RECEPTORA I AGONISTA BETA-2 ADRENORECEPTORA (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 1087
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Patent: 201407864U
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 06844
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1203890
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1742140
Estimated Expiration: ⤷ Sign Up
Patent: 1830728
Estimated Expiration: ⤷ Sign Up
Patent: 120092163
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Sign Up
Patent: 170061719
Patent: 무스카린성 수용체 길항제 및 베타-2 아드레날린 수용체 효능제의 조합물 (- COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Sign Up
Patent: 180128937
Patent: 질환을 치료하는데 유용한 FIMH의 만노스-유래 길항제
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 59330
Estimated Expiration: ⤷ Sign Up
Turkey
Patent: 1802921
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 6775
Patent: КОМБІНАЦІЯ АНТАГОНІСТА МУСКАРИНОВОГО РЕЦЕПТОРА ТА АГОНІСТА БЕТА-2 АДРЕНОРЕЦЕПТОРА (COMBINATIONS OF MUSCARINIC ACETYLCHOLINE RECEPTOR ANTAGONIST AND BETA 2 AGONIST)
Estimated Expiration: ⤷ Sign Up
United Kingdom
Patent: 21075
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ANORO ELLIPTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1730676 | COMPTEUR A UTILISER AVEC UN DISTRIBUTEUR DE MEDICAMENT (COUNTER FOR USE WITH A MEDICAMENT DISPENSER) | ⤷ Sign Up |
| Spain | 2316599 | ⤷ Sign Up | |
| Japan | 2009518093 | ⤷ Sign Up | |
| Mexico | 351290 | COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2. (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST.) | ⤷ Sign Up |
| Portugal | 1907037 | ⤷ Sign Up | |
| Japan | H04220266 | INHALER | ⤷ Sign Up |
| Russian Federation | 2004106555 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ANORO ELLIPTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1425001 | CA 2014 00021 | Denmark | ⤷ Sign Up | PRODUCT NAME: VILANTEROL ELLER ET SALT ELLER SOLVAT DERAF, HERUNDER VILANTEROL TRIFENATAT; REG. NO/DATE: EU/1/13/886/001-006 20131113 |
| 2506844 | PA2018011,C2506844 | Lithuania | ⤷ Sign Up | PRODUCT NAME: UMEKLIDINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA (PVZ., UMEKLIDINO BROMIDAS), VILANTEROLIS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA (PVZ., VILANTEROLIO TRIFENATATAS) IR FLUTIKAZONO FUROATAS; REGISTRATION NO/DATE: EU/1/17/1236 20171115 |
| 1425001 | C300664 | Netherlands | ⤷ Sign Up | PRODUCT NAME: VILANTEROL, DAN WEL EEN ZOUT OF SOLVAAT DAARVAN, IN HET BIJZONDER HET TRIFENYLACETAATZOUT; REGISTRATION NO/DATE: EU/1/13/886/001-006 20131113 |
| 2506844 | PA2018011 | Lithuania | ⤷ Sign Up | PRODUCT NAME: UMEKLIDINAS + VILANTEROLIS + FLUTIKAZONO FUROATAS; REGISTRATION NO/DATE: EU/1/17/1236 20171115 |
| 1425001 | 174 50004-2014 | Slovakia | ⤷ Sign Up | PRODUCT NAME: VILANTEROLTRIFENATAT; REGISTRATION NO/DATE: EU/1/13/886/001 - EU/1/13/886/006 20131113 |
| 1740177 | PA2014038,C1740177 | Lithuania | ⤷ Sign Up | PRODUCT NAME: UMEKLIDINO BROMIDAS; REGISTRATION NO/DATE: EU/1/14/922 20140428 |
| 1425001 | 2014/024 | Ireland | ⤷ Sign Up | PRODUCT NAME: VILANTEROL OR A SALT OR SOLVATE THEREOF; REGISTRATION NO/DATE: EU/1/13/886/001-006 20131113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


