ANORO ELLIPTA Drug Patent Profile
✉ Email this page to a colleague
When do Anoro Ellipta patents expire, and what generic alternatives are available?
Anoro Ellipta is a drug marketed by Glaxosmithkline and is included in one NDA. There are nine patents protecting this drug.
This drug has two hundred and fifty-four patent family members in fifty-one countries.
The generic ingredient in ANORO ELLIPTA is umeclidinium bromide; vilanterol trifenatate. There are two drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the umeclidinium bromide; vilanterol trifenatate profile page.
DrugPatentWatch® Generic Entry Outlook for Anoro Ellipta
Anoro Ellipta was eligible for patent challenges on May 10, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 29, 2030. This may change due to patent challenges or generic licensing.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ANORO ELLIPTA?
- What are the global sales for ANORO ELLIPTA?
- What is Average Wholesale Price for ANORO ELLIPTA?
Summary for ANORO ELLIPTA
| International Patents: | 254 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 57 |
| Clinical Trials: | 49 |
| Patent Applications: | 516 |
| Drug Prices: | Drug price information for ANORO ELLIPTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ANORO ELLIPTA |
| What excipients (inactive ingredients) are in ANORO ELLIPTA? | ANORO ELLIPTA excipients list |
| DailyMed Link: | ANORO ELLIPTA at DailyMed |
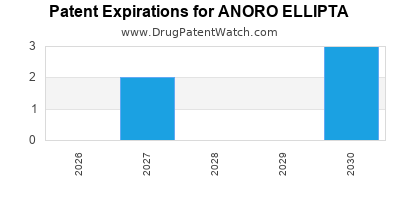
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ANORO ELLIPTA
Generic Entry Date for ANORO ELLIPTA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INHALATION |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ANORO ELLIPTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| GlaxoSmithKline | PHASE4 |
| University of Tennessee Graduate School of Medicine | PHASE4 |
| Theravance Biopharma | PHASE4 |
Pharmacology for ANORO ELLIPTA
| Drug Class | Anticholinergic beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists Cholinergic Antagonists |
US Patents and Regulatory Information for ANORO ELLIPTA
ANORO ELLIPTA is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ANORO ELLIPTA is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ANORO ELLIPTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| Glaxosmithkline | ANORO ELLIPTA | umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 203975-001 | Dec 18, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ANORO ELLIPTA
When does loss-of-exclusivity occur for ANORO ELLIPTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10326798
Patent: Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Estimated Expiration: ⤷ Get Started Free
Patent: 14204459
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Patent: 16262698
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Patent: 17238392
Patent: Mannose-derived antagonists of FimH useful for treating disease
Estimated Expiration: ⤷ Get Started Free
Patent: 18282427
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Patent: 21204302
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Patent: 23219901
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012012925
Patent: produto de combinação farmacêutico, inalador de pó seco, e, uso de um produto
Estimated Expiration: ⤷ Get Started Free
Patent: 2018069147
Patent: composto da fórmula iii, composição farmacêutica, método de inibição da função de fimh e método de tratamento de uma doença mediada por fimh
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 81487
Patent: COMBINAISONS D'UN ANTAGONISTE DE RECEPTEUR MUSCARINIQUE ET D'UN AGONISTE DU RECEPTEUR BETA-2 ADRENERGIQUE (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 18345
Patent: ANTAGONISTES DERIVES DU MANNOSE DE FIMH UTILES POUR LE TRAITEMENT D'UNE MALADIE (MANNOSE-DERIVED ANTAGONISTS OF FIMH USEFUL FOR TREATING DISEASE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12001432
Patent: Producto de combinacion farmacéutica que comprende bromuro de 4-[hidroxi-(difenil)-metil]-1-{2-[(fenil-metil)-oxi]-etil}-1-azonia-biciclo-[2.2.2]-octano y trifenil-acetato de 4-{(1r)-2-[(6-{2-[(2,6-dicloro-bencil)-oxi]-etoxi}-hexil)-amino]-1-hidroxi-etil}-2-(hidroxi-metil)-fenol; y su uso para tratar enfermedades respiratorias.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2724974
Patent: Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Estimated Expiration: ⤷ Get Started Free
Patent: 7412229
Patent: 毒蕈碱受体拮抗剂和β‑2肾上腺素受体激动剂的组合 (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 8778288
Patent: 可用于治疗疾病的FimH的甘露糖衍生的拮抗剂 (Mannose-derived antagonists of FimH useful for treating disease)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 41613
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA 2
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 120265
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0180312
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20058
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 06844
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 012000148
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 3839
Patent: КОМБИНАЦИИ АНТАГОНИСТА МУСКАРИНОВЫХ РЕЦЕПТОРОВ И АГОНИСТА БЕТА-2-АДРЕНОРЕЦЕПТОРОВ (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 1290266
Patent: КОМБИНАЦИИ АНТАГОНИСТА МУСКАРИНОВЫХ РЕЦЕПТОРОВ И АГОНИСТА БЕТА-2-АДРЕНОРЕЦЕПТОРОВ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 06844
Patent: Combinaisons d'un antagoniste de récepteur muscarinique et d'un agoniste du récepteur bêta-2 adrénergique (Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist)
Estimated Expiration: ⤷ Get Started Free
Patent: 35707
Patent: COMBINAISONS D'UN ANTAGONISTE DE RÉCEPTEUR MUSCARINIQUE ET D'UN AGONISTE DU RÉCEPTEUR BÊTA-2 ADRÉNERGIQUE (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 32892
Patent: ANTAGONISTES DÉRIVÉS DU MANNOSE DE FIMH UTILES POUR LE TRAITEMENT D'UNE MALADIE (MANNOSE-DERIVED ANTAGONISTS OF FIMH USEFUL FOR TREATING DISEASE)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1022
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 49407
Patent: 毒蕈碱受體拮抗劑和β-2腎上腺素受體激動劑的組合 (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 36216
Estimated Expiration: ⤷ Get Started Free
Patent: 800027
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9893
Patent: שילובים של אנטגוניסט לקולטן מוסקריני ואגוניסט לקולטן בתא-2-אדרנו (Combinations of a muscarinic receptor antagonist and a beta-2 adrenorecptor agonist)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 16631
Estimated Expiration: ⤷ Get Started Free
Patent: 13512270
Estimated Expiration: ⤷ Get Started Free
Patent: 19509315
Patent: 疾患の処置に有用なFimHのマンノース由来アンタゴニスト
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 506844
Estimated Expiration: ⤷ Get Started Free
Patent: 2018011
Estimated Expiration: ⤷ Get Started Free
Patent: 06844
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0077
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 4864
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1290
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARÍNICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2. (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST.)
Estimated Expiration: ⤷ Get Started Free
Patent: 12006310
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2. (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 965
Patent: KOMBINACIJE ANTAGONISTA MUSKARINSKOG RECEPTORA I AGONISTA ВЕТА - 2 ADRENORECEPTORA (Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 853
Patent: تركيبة مضاد استقبال المسكارينية ومحضر استقبال بتي ـ 2 الأدرينالية
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0026
Patent: Combinations of a muscarinic receptor antagonist and a beta-2 adrenoreceptor agonist
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 06844
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 130042
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Get Started Free
Patent: 170915
Patent: COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 06844
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 06844
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 18131440
Patent: Антагонисты FimH, являющиеся производными маннозы, пригодные для лечения заболевания
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 848
Patent: KOMBINACIJE ANTAGONISTA MUSKARINSKOG RECEPTORA I AGONISTA BETA-2 ADRENORECEPTORA (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 1087
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Patent: 201407864U
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 06844
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1203890
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1742140
Estimated Expiration: ⤷ Get Started Free
Patent: 1830728
Estimated Expiration: ⤷ Get Started Free
Patent: 120092163
Patent: COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST
Estimated Expiration: ⤷ Get Started Free
Patent: 170061719
Patent: 무스카린성 수용체 길항제 및 베타-2 아드레날린 수용체 효능제의 조합물 (- COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 180128937
Patent: 질환을 치료하는데 유용한 FIMH의 만노스-유래 길항제
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 59330
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1802921
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 6775
Patent: КОМБІНАЦІЯ АНТАГОНІСТА МУСКАРИНОВОГО РЕЦЕПТОРА ТА АГОНІСТА БЕТА-2 АДРЕНОРЕЦЕПТОРА (COMBINATIONS OF MUSCARINIC ACETYLCHOLINE RECEPTOR ANTAGONIST AND BETA 2 AGONIST)
Estimated Expiration: ⤷ Get Started Free
United Kingdom
Patent: 21075
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ANORO ELLIPTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20170061719 | 무스카린성 수용체 길항제 및 베타-2 아드레날린 수용체 효능제의 조합물 (- COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST) | ⤷ Get Started Free |
| South Africa | 200608565 | MUSCARINIC ACETYLCHOLINE RECEPTOR ANTAGONISTS | ⤷ Get Started Free |
| Mexico | 2012006310 | COMBINACIONES DE UN ANTAGONISTA DE LOS RECEPTORES MUSCARINICOS Y UN AGONISTA DEL ADRENO-RECEPTOR BETA-2. (COMBINATIONS OF A MUSCARINIC RECEPTOR ANTAGONIST AND A BETA-2 ADRENORECEPTOR AGONIST.) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ANORO ELLIPTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1740177 | C01740177/02 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: UMECLIDINIUM BROMID + VILANTEROL; REGISTRATION NO/DATE: SWISSMEDIC 63152 14.07.2014 |
| 1740177 | 208 50012-2014 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: UMEKLIDINIUMBROMID; REGISTRATION NO/DATE: EU/1/14/922/001 - EU/1/14/922/003 20140430 |
| 1425001 | C 2014 016 | Romania | ⤷ Get Started Free | PRODUCT NAME: VILANTEROL SAU O SARE SAU SOLVAT AL ACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/13/886/001, EU/1/13/886/002, EU/1/13/886/003, EU/1/13/886/004, EU/1/13/886/005, EU/1/13/886/006; DATE OF NATIONAL AUTHORISATION: 20131113; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/886/001, EU/1/13/886/002, EU/1/13/886/003, EU/1/13/886/004, EU/1/13/886/005, EU/1/13/886/006; DATE OF FIRST AUTHORISATION IN EEA: 20131113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ANORO ELLIPTA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
