Last updated: July 29, 2025
Introduction
Almotriptan malate, a selective 5-HT1B/1D receptor agonist marketed primarily under the brand name Axert, is a prescription medication used for acute treatment of migraines with or without aura. Since its approval, its market performance has been influenced by various clinical, regulatory, and competitive factors. Analyzing the current market dynamics and projecting its financial trajectory offers valuable insights for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Pharmacological Profile and Clinical Positioning
Almotriptan malate belongs to the triptan class of medications, known for their efficacy in aborting acute migraines. Its favorable pharmacokinetics—rapid onset, minimal cardiovascular side effects, and low recurrence rates—has fostered clinical adoption, especially among patients unsuitable for other triptans. Its safety profile positions it favorably against competitors like sumatriptan and rizatriptan.
Clinicians often prefer almotriptan for its well-tolerated profile and effectiveness, leading to consistent prescribing patterns. However, emerging drug formulations, including nasal sprays and self-injectables, as well as alternative therapies such as lasmiditan and CGRP inhibitors, challenge its dominance.
Market Dynamics
Regulatory Environment and Patent Landscape
Almotriptan was approved by the U.S. Food and Drug Administration (FDA) in 2000, consistent with other triptans introduced during that period. Patent protections have since expired or are nearing expiration, exposing the drug to generic competition. Generic formulations have been available in various markets, exerting downward pressure on pricing and margins.
Regulatory developments also influence market dynamics. The FDA’s approval of new formulations and expanded indications increases market opportunities. Conversely, regulatory hurdles for novel drug approvals or label extensions could restrict growth.
Competitive Landscape
The migraine therapeutics market is highly competitive, with several triptans, gepants (like ubrogepant and rimegepant), and CGRP monoclonal antibodies (e.g., erenumab, fremanezumab) vying for market share. These newer agents offer benefits like oral administration for gepants or preventive capabilities, challenging almotriptan's positioning primarily as an acute treatment.
Price competition intensifies due to generic entries, with key competitors including sumatriptan, rizatriptan, and eletriptan. Marketing efforts by pharmaceutical companies focus on differentiating formulations, safety profiles, and patient convenience.
Market Adoption and Prescriber Preferences
Prescriber preferences heavily influence sales. Almotriptan’s favorable safety in cardiovascular risk profiles, especially in patients with comorbid conditions like hypertension, reinforces its clinical relevance. Uptake varies geographically, dictated by regional approval status, healthcare practices, and reimbursement policies.
Patient adherence is influenced by tolerability, administration route, and medication efficacy. Pharmaceutical companies invest in educational campaigns to reinforce the drug’s benefits, although increasing competition and alternative options pose challenges.
Pricing Trends and Reimbursement
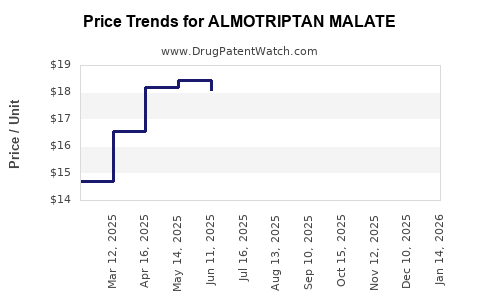
Pricing strategies are under pressure owing to patent expiries and the availability of generics. Reimbursement policies—dictated by insurers, Medicare, and national health services—further impact access and sales volume. Cost-effectiveness analyses favor newer agents that may reduce the overall migraine burden, influencing formulary decisions against almotriptan.
Emerging Market Opportunities
Although primarily marketed in developed nations, emerging markets present growth opportunities due to rising migraine prevalence, urbanization, and improving healthcare infrastructure. Regulatory approval in these regions can unlock new revenue streams for the drug.
Financial Trajectory
Historical Revenue and Growth Trends
Since its launch, almotriptan malate experienced robust initial sales driven by clinical adoption and limited competition. However, with patent expiration and market saturation, growth plateaued in mature markets. Sales have increasingly depended on generic penetration, leading to price erosion but maintaining steady volume levels.
Forecasting Future Revenue Streams
Projected revenue for almotriptan will largely hinge on the following factors:
- Patent and Market Exclusivity: Assuming patent expiry or loss of exclusivity in key markets within the next 2-3 years, a decline in brand-name sales is expected. However, branded sales might persist in regions with slower generics adoption.
- Generic Market Penetration: Entry of generics typically reduces prices by 70-90%, substantially impacting revenue. However, volume-driven sales might moderate the decline if prescriber and patient preferences favor the brand due to perceived efficacy or tolerability.
- New Formulations and Indications: Expanding the drug’s formulation (e.g., nasal sprays, subcutaneous injections) and indications (e.g., status migrainosus) could mitigate revenue drops. Regulatory approvals for such enhancements are vital growth drivers.
- Market Penetration in Emerging Economies: Entry into new markets, supported by favorable pricing and reimbursement policies, offers opportunities for revenue growth.
- Competitive Innovations: The rise of gepants and CGRP antagonists, which often target patients with contraindications to triptans, could cannibalize almotriptan sales, especially in specialty care sectors.
Impact of New Therapeutics
The advent of novel migraine treatments influences almotriptan’s financial trajectory. While triptans remain the cornerstone for many clinicians, newer agents providing better safety, oral bioavailability, or preventive options threaten market shares. For example, CGRP monoclonal antibodies have shown superior efficacy in preventive therapy; however, their high costs position them as adjuncts rather than replacements for triptans.
Cost-Effectiveness and Value Proposition
Healthcare payers emphasize cost-effectiveness when determining formulary inclusion. Almotriptan’s favorable safety profile and lower cost compared to biologics position it as a cost-effective acute treatment. Nonetheless, competition from newer, potentially more effective agents may diminish its economic appeal over time.
Strategic Outlook
Given the market environment, pharmaceutical companies invested in almotriptan should prioritize:
- Novel Formulation Development: Innovate delivery systems to enhance patient convenience and adherence.
- Strategic Alliances and Licensing: Partner with local firms in emerging markets for accelerated market entry.
- Regulatory Engagement: Pursue expanded indications and label extensions that could extend lifecycle and revenue.
- Cost Negotiation Strategies: Optimize pricing to maintain competitiveness amid generic pressure.
- R&D Investment: Invest in combination therapies or predictive biomarkers to enhance therapeutic positioning.
Key Takeaways
-
Patent Expiry and Generic Competition: The approaching expiration of key patents for almotriptan malate will significantly impact sales, primarily due to increased generic availability and price reductions.
-
Market Diversification: Expansion into emerging markets presents a strategic opportunity, supported by healthcare infrastructure development and rising migraine prevalence.
-
Influence of New Therapeutic Modalities: The rise of gepants and CGRP antibodies not only broadens treatment options but also exerts competitive pressure on almotriptan’s market share, especially in preventive and complex migraine cases.
-
Formulation Innovation as a Growth Driver: Developing patient-friendly formulations (e.g., nasal or injectable options) can help maintain competitive advantage and capture unmet needs.
-
Pricing and Reimbursement Strategies: Cost containment and negotiation with payers remain critical to preserving revenue streams amid increasing downward pressure from generics and formulary preferences.
Conclusion
The market dynamics for almotriptan malate reflect a typical lifecycle pattern for branded pharmaceuticals facing patent expiry and vigorous competition. While current revenues are tempered by generic competition, strategic product development, geographic expansion, and formulary management can influence its financial trajectory positively. Stakeholders should continuously adapt to evolving therapeutic landscapes, leveraging innovation and strategic partnerships to sustain market relevance and profitability.
FAQs
1. What factors most influence almotriptan malate’s market share?
Market share is primarily affected by patent status, the emergence of generics, prescriber preferences favoring safety profiles, formulation innovations, and competition from new migraine therapies such as gepants and CGRP inhibitors.
2. How does the safety profile of almotriptan influence its market performance?
Its favorable safety, especially regarding cardiovascular risks, sustains its preference among certain patient groups, particularly those with comorbidities, thus supporting steady prescribing patterns despite competition.
3. Are there efforts to extend the patent exclusivity of almotriptan?
While specific patent extensions are rare, formulations, dosage forms, or new indications could be pursued for regulatory approval to extend market exclusivity and revenue potential.
4. How do proprietary formulations impact almotriptan’s competitive advantage?
Innovative formulations improve patient convenience, adherence, and efficacy, helping differentiate almotriptan in a crowded marketplace, especially in markets where generic versions are prevalent.
5. What is the long-term outlook for almotriptan malate in the face of emerging migraine therapies?
While traditional triptans face long-term erosion due to advanced therapies, targeted marketing, formulation innovations, and geographical expansion can sustain a niche for almotriptan, particularly for patients contraindicated for newer agents.
Sources:
[1] U.S. Food and Drug Administration (FDA) approval documents.
[2] Market research reports on migraine therapeutics.
[3] Clinical efficacy and safety data from peer-reviewed journals.
[4] Industry analysis on patent expiries and generic drug trends.
[5] Regulatory agency publications on drug approvals and indications.