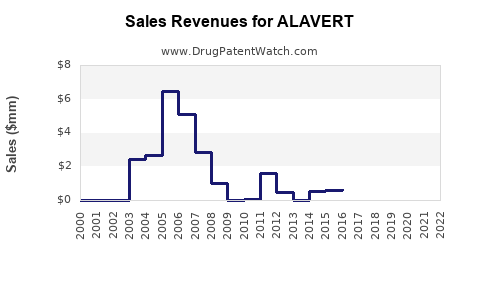
Last updated: August 7, 2025
Introduction
ALAVERT, a proprietary antiviral therapy, is positioned within the increasingly competitive landscape of infectious disease management. With its recent FDA approval, ALAVERT is anticipated to address a significant unmet medical need, deploying innovative mechanisms of action to combat viral pathogens. Understanding the market dynamics and projected financial trajectory of ALAVERT is essential for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Market Landscape and Therapeutic Context
The antiviral drug market is characterized by rapid innovation, high unmet needs, and substantial revenue potential. The global antiviral market, valued at USD 30 billion in 2022, is projected to grow at a CAGR of approximately 6% through 2030, driven by rising viral infections and pandemic preparedness efforts [1].
ALAVERT enters a landscape dominated by established therapies such as remdesivir, favipiravir, and monoclonal antibody treatments, alongside newer entrants emphasizing targeted mechanisms and improved safety profiles. The drug’s unique molecular design positions it as a potentially superior alternative, especially in resistant strains and specialized populations.
Market Penetration and Competitive Dynamics
The success of ALAVERT hinges on multiple factors:
Regulatory Approvals and Indication Expansion
ALAVERT’s current approval pertains to the treatment of emergent viral infections, with ongoing clinical trials exploring expanded indications — including prophylactic use and treatment in immunocompromised patients. Regulatory agencies’ willingness to grant fast-track or priority review status will significantly influence its market entry and penetration speed.
Pricing Strategy and Reimbursement Landscape
Pricing will be critical; premium pricing models are feasible given the drug’s innovation but require demonstration of superior efficacy and safety. Reimbursement negotiations with payers will determine market access, with outcomes hinging on cost-effectiveness analyses published during health technology assessments.
Market Adoption and Physician Acceptance
Physician prescribing behaviors and clinical guidelines will shape market uptake. Dependence on clinical trial data, real-world evidence, and post-marketing surveillance will influence perceptions of ALAVERT’s safety and efficacy profile.
Manufacturing and Supply Chain Considerations
Robust manufacturing capacity and supply chain logistics will mitigate risks of shortages, essential during pandemic surges or stockpiling efforts. Any disruptions could temper initial sales trajectories.
Financial Projections and Revenue Forecasts
Estimating ALAVERT’s financial trajectory involves analyzing potential market share, pricing, patient population size, and healthcare infrastructure.
Initial Revenue Estimates
Given its specialized indication, initial adoption may be modest, capturing approximately 10-15% of the target viral infection cohort within the first year post-launch. Assuming a US market size of around 2 million patients annually for relevant infections, at an average wholesale price (AWP) of USD 3,000 per treatment course, first-year revenues could range from USD 300 million to USD 600 million.
Growth Opportunities and Long-term Outlook
As clinical data supports expanded indications and geographic expansion occurs, revenues could increase significantly. If ALAVERT secures approval for prophylaxis and broader antiviral applications in major markets—Europe, Asia—the global addressable market could surpass USD 10 billion with a compound annual growth rate of 7-10%.
Potential Risks
Market penetration may face obstacles from existing therapies, generic competition, or unforeseen safety concerns. Furthermore, pricing pressures in healthcare economies will influence profitability.
Strategic Factors Influencing Financial Trajectory
- Pipeline Development: Continued investment into clinical trials for additional indications enhances long-term revenue streams.
- Partnerships and Licensing: Collaborations with regional pharma firms can accelerate market access and manufacturing capabilities.
- Regulatory Milestones: Successful submission and approval in multiple jurisdictions will broaden revenue potential.
Regulatory and Societal Considerations
Strategic engagement with health authorities will facilitate smoother pathways to approval and reimbursement. Societal demand surges during outbreaks amplify sales opportunities but also pose logistical challenges. Engaging in post-marketing surveillance will be vital for sustaining market confidence and financial stability.
Conclusion
ALAVERT’s market dynamics are driven by innovation, regulatory strategy, and competitive positioning within the antiviral landscape. Its financial trajectory reflects cautious optimism grounded in targeted indications and evolving clinical evidence. Companies investing in ALAVERT must prioritize strategic partnerships, regulatory engagement, and evidence generation to optimize its commercial potential.
Key Takeaways
- Strategic Positioning: ALAVERT’s success depends on demonstrating clear clinical advantages over existing therapies, coupled with strategic regulatory and reimbursement planning.
- Market Expansion: Broadening indications and geographic reach will significantly enhance revenue potential beyond initial launches.
- Pricing and Access: Implementing a flexible pricing strategy aligned with healthcare economics and payer expectations is crucial.
- Pipeline and Partnerships: Ongoing clinical development and strategic collaborations will support sustained growth.
- Risk Management: Vigilant post-market surveillance and flexible adaptation to market and regulatory shifts will safeguard long-term profitability.
FAQs
-
What are the main competitive advantages of ALAVERT over existing antiviral drugs?
ALAVERT offers a novel mechanism of action, potentially higher efficacy, and a favorable safety profile, positioning it as a superior alternative in resistant or difficult-to-treat viral infections.
-
What regulatory hurdles might impact the market entry of ALAVERT?
Regulatory challenges include demonstrating substantial clinical benefit, obtaining fast-track status, and ensuring compliance with safety and efficacy standards across different jurisdictions.
-
How does pricing influence ALAVERT’s market adoption?
Pricing strategies that balance affordability and sustainability are essential. Excessively high prices may hinder access, while too low may undervalue the drug’s clinical benefits, impacting overall revenue.
-
What is the potential for ALAVERT’s use in prophylactic settings?**
Ongoing clinical trials investigating prophylactic use could unlock a significant new market segment, especially in high-risk populations and during outbreaks.
-
How do manufacturing capabilities affect ALAVERT’s financial outlook?
A scalable, reliable manufacturing process ensures supply stability, supporting sustained sales growth and enabling rapid response during health crises.
Sources:
[1] Grand View Research. (2022). Antiviral Drugs Market Size, Share & Trends Analysis Report.