AKYNZEO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Akynzeo, and what generic alternatives are available?
Akynzeo is a drug marketed by Helsinn Hlthcare and is included in two NDAs. There are twenty-two patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and ninety patent family members in fifty-three countries.
The generic ingredient in AKYNZEO is fosnetupitant chloride hydrochloride; palonosetron hydrochloride. There is one drug master file entry for this compound. Additional details are available on the fosnetupitant chloride hydrochloride; palonosetron hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Akynzeo
Akynzeo was eligible for patent challenges on April 19, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 25, 2035. This may change due to patent challenges or generic licensing.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AKYNZEO?
- What are the global sales for AKYNZEO?
- What is Average Wholesale Price for AKYNZEO?
Summary for AKYNZEO
| International Patents: | 190 |
| US Patents: | 22 |
| Applicants: | 1 |
| NDAs: | 2 |
| Clinical Trials: | 11 |
| Drug Prices: | Drug price information for AKYNZEO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AKYNZEO |
| What excipients (inactive ingredients) are in AKYNZEO? | AKYNZEO excipients list |
| DailyMed Link: | AKYNZEO at DailyMed |
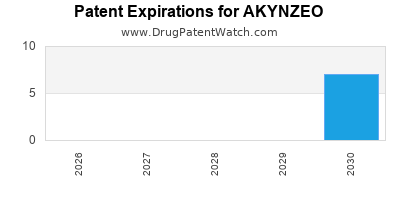

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AKYNZEO
Generic Entry Dates for AKYNZEO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for AKYNZEO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for AKYNZEO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Fondazione IRCCS Istituto Nazionale dei Tumori, Milano | PHASE4 |
| Simon Williamson Clinic | Phase 2 |
| Helsinn Healthcare SA | Phase 2 |
Pharmacology for AKYNZEO
Paragraph IV (Patent) Challenges for AKYNZEO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AKYNZEO | Solution in SDV | fosnetupitant chloride hydrochloride; palonosetron hydrochloride | 235 mg/0.25 mg per 20 mL | 210493 | 1 | 2022-04-19 |
US Patents and Regulatory Information for AKYNZEO
AKYNZEO is protected by twenty-four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AKYNZEO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for AKYNZEO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Helsinn Hlthcare | AKYNZEO | netupitant; palonosetron hydrochloride | CAPSULE;ORAL | 205718-001 | Oct 10, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Helsinn Hlthcare | AKYNZEO | netupitant; palonosetron hydrochloride | CAPSULE;ORAL | 205718-001 | Oct 10, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AKYNZEO
When does loss-of-exclusivity occur for AKYNZEO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8676
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 15323515
Patent: Crystalline forms of an NK-1 antagonist
Estimated Expiration: ⤷ Get Started Free
Patent: 17276588
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2018074655
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 60599
Patent: FORMES CRISTALLINES D'UN ANTAGONISTE DES RECEPTEURS NK-1 (CRYSTALLINE FORMS OF AN NK-1 ANTAGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 25837
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 18003338
Estimated Expiration: ⤷ Get Started Free
China
Patent: 7001275
Patent: NK‑1拮抗剂的晶型 (Crystalline forms of an NK-1 antagonist)
Estimated Expiration: ⤷ Get Started Free
Patent: 9310627
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 18011686
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0200196
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 22755
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 19000169
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6605
Estimated Expiration: ⤷ Get Started Free
Patent: 1892837
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 97871
Patent: FORMES CRYSTALLINES D'UN ANTAGONIST DE NK-1 (CRYSTALLINE FORMS OF AN NK-1 ANTAGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
Patent: 26231
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3126
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 36817
Estimated Expiration: ⤷ Get Started Free
Patent: 23084
Estimated Expiration: ⤷ Get Started Free
Patent: 17529380
Patent: NK−1アンタゴニストの結晶形態
Estimated Expiration: ⤷ Get Started Free
Patent: 19521102
Estimated Expiration: ⤷ Get Started Free
Patent: 21183639
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0170137
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 4081
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6118
Estimated Expiration: ⤷ Get Started Free
Patent: 18015036
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 513
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7865
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 190347
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 018502524
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 852
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201809708P
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 35980
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1807932
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2459416
Estimated Expiration: ⤷ Get Started Free
Patent: 170063768
Patent: NK-1 길항제의 결정질 형태 (-1 CRYSTALLINE FORMS OF AN NK-1 ANTAGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 190015276
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 71226
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 25191
Estimated Expiration: ⤷ Get Started Free
Patent: 1613888
Patent: Crystalline forms of an NK-1 antagonist
Estimated Expiration: ⤷ Get Started Free
Patent: 1801727
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 2285
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 273
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AKYNZEO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2016147868 | NK−1レセプター関連疾患の治療のための置換4−フェニル−ピリジン (SUBSTITUTED 4-PHENYL PYRIDINES FOR TREATING NK-1 RECEPTOR ASSOCIATED DISEASES) | ⤷ Get Started Free |
| Malaysia | 194081 | PHYSIOLOGICALLY BALANCED INJECTABLE FORMULATIONS OF FOSNETUPITANT | ⤷ Get Started Free |
| Italy | 1320763 | DERIVATI DELLA 4-FENIL-PIRIDINA. | ⤷ Get Started Free |
| South Korea | 20120101456 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AKYNZEO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2785706 | PA2020510,C2785706 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: FOSNETUPITANTAS; REGISTRATION NO/DATE: EU/1/15/1001 20200316 |
| 2785706 | C20200029 00371 | Estonia | ⤷ Get Started Free | PRODUCT NAME: FOSNETUPITANT;REG NO/DATE: EU/1/15/1001; 18.03.2020 |
| 2785706 | 20C1029 | France | ⤷ Get Started Free | PRODUCT NAME: FOSNETUPITANT OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES, EN PARTICULIER LE SEL DE CHLORHYDRATE DE CHLORURE DE FOSNETUPITANT; REGISTRATION NO/DATE: EU/1/15/1001 20200318 |
| 2785706 | C02785706/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: FOSNETUPITANT, PALONOSETRON.; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 68290 01.09.2022 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for AKYNZEO (Netupitant/Palonosetron)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
