ACANYA Drug Patent Profile
✉ Email this page to a colleague
When do Acanya patents expire, and when can generic versions of Acanya launch?
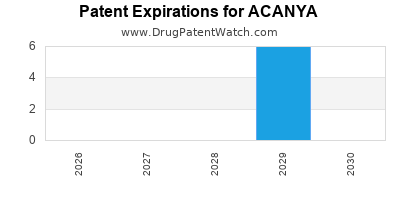
Acanya is a drug marketed by Bausch and is included in one NDA. There are six patents protecting this drug.
This drug has twenty patent family members in fourteen countries.
The generic ingredient in ACANYA is benzoyl peroxide; clindamycin phosphate. There are seventeen drug master file entries for this compound. Fourteen suppliers are listed for this compound. Additional details are available on the benzoyl peroxide; clindamycin phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Acanya
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
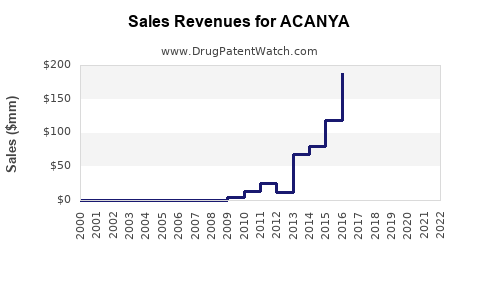
Summary for ACANYA
| International Patents: | 20 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 6 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ACANYA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ACANYA |
| What excipients (inactive ingredients) are in ACANYA? | ACANYA excipients list |
| DailyMed Link: | ACANYA at DailyMed |

Recent Clinical Trials for ACANYA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Taro Pharmaceuticals USA | Phase 1 |
| Watson Laboratories, Inc. | Phase 3 |
| Padagis LLC | Phase 3 |
Pharmacology for ACANYA
| Drug Class | Lincosamide Antibacterial |
| Physiological Effect | Decreased Sebaceous Gland Activity |
Anatomical Therapeutic Chemical (ATC) Classes for ACANYA
US Patents and Regulatory Information for ACANYA
ACANYA is protected by six US patents.
Patents protecting ACANYA
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ACANYA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ACANYA
When does loss-of-exclusivity occur for ACANYA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09255679
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0913326
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 23029
Estimated Expiration: ⤷ Try a Trial
China
Patent: 2056481
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0200450
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 99810
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 77693
Estimated Expiration: ⤷ Try a Trial
Patent: 06272
Estimated Expiration: ⤷ Try a Trial
Patent: 11522820
Estimated Expiration: ⤷ Try a Trial
Patent: 15038093
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 10013152
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 99810
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 93847
Estimated Expiration: ⤷ Try a Trial
Patent: 45087
Estimated Expiration: ⤷ Try a Trial
Patent: 10146038
Estimated Expiration: ⤷ Try a Trial
Patent: 13122395
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1008265
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 110014651
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 73931
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ACANYA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Brazil | PI0913326 | formulações farmacêuticas tópicas contendo uma baixa concentração de peróxido de benzoíla em suspensão aquosa e um solvente orgânico miscível em água | ⤷ Try a Trial |
| Japan | 6006272 | ⤷ Try a Trial | |
| Norway | 308155 | ⤷ Try a Trial | |
| Russian Federation | 2122412 | КОМПОЗИЦИИ КЛИНДАМИЦИНА И БЕНЗОИЛПЕРОКСИДА ДЛЯ ЛЕЧЕНИЯ АКНЕ, НАБОР ДЛЯ ПРИГОТОВЛЕНИЯ КОМПОЗИЦИИ, СПОСОБ ПОЛУЧЕНИЯ КОМПОЗИЦИИ, СПОСОБ ЛЕЧЕНИЯ АКНЕ (COMPOSITION OF CLINDAMYCIN AND BENZOYL PEROXIDE FOR ACNE TREATMENT, A SET FOR COMPOSITION PREPARING, A METHOD OF COMPOSITION PREPARING, METHOD OF TREATMENT OF PATIENTS WITH ACNE) | ⤷ Try a Trial |
| South Korea | 20110014651 | TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING A LOW CONCENTRATION OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND A WATER-MISCIBLE ORGANIC SOLVENT | ⤷ Try a Trial |
| Singapore | 48195 | Compositions of clyndamycin and benzoyl peroxide for acne treatment | ⤷ Try a Trial |
| Denmark | 0626844 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ACANYA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1458369 | C01458369/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: ADAPALENUM + BENZOYLIS PEROXIDUM; REGISTRATION NUMBER/DATE: SWISSMEDIC 58460 19.05.2009 |
| 1586316 | SPC/GB11/054 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC 2-AMINO-3-(4-BROMOBENZOYL)PHENYLACETIC ACID OR A PHARMACOLOGICALLY ACCEPTABLE SALT THEREOF OR A HYDRATE THEREOF; REGISTERED: UK EU/1/11/692/001 20110523 |
| 0591275 | SPC/GB05/030 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NITISINONE (2-(2-NITRO-4-TRIFLUOROMETHYLBENZOYL)-1,3-CYCLOHEXANEDIONE) OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/04/303/001 20050221; UK EU/1/04/303/002 20050221; UK EU/1/04/303/003 20050221 |
| 1458369 | CA 2008 00029 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ADAPALEN, BENZOYLPEROXID |
| 1458369 | 122008000041 | Germany | ⤷ Try a Trial | PRODUCT NAME: ADAPALEN IN KOMBINATION MIT BENZOYLPEROXID; NAT. REGISTRATION NO/DATE: 67913.00.00 20080229; FIRST REGISTRATION: DAENEMARK 40440 20071218 |
| 1667986 | 92172 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| 0526708 | C300097 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: BOSENTAN, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN HYDRAAT OF IN DE VORM VAN EEN ESTER VAN DE HYDROXYLGROEP VAN DE 2-HYDROXYETHOXY REST MET EEN ZUUR MET DE FORMULE R5-OH, WAARIN R5 EEN C1-7-ALKANOYL, BENZOYL, OF HETEROCYCLYCARBONYL VOORSTELT; NATL. REGISTRATION NO/DATE: U/1/02/220/001 - 005 20020515; FIRST REGISTRATION: CH IKS 58841 01 - 02 20020228 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


