Share This Page
Drug Sales Trends for ACANYA
✉ Email this page to a colleague
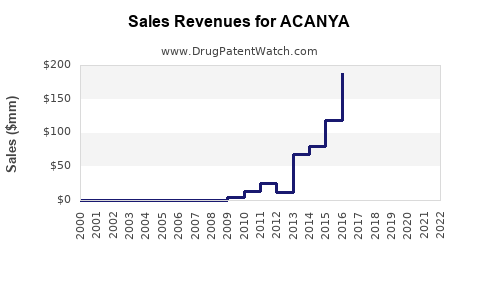
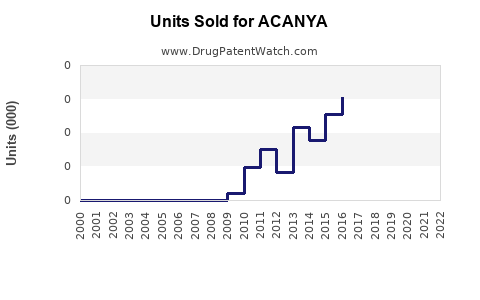
Annual Sales Revenues and Units Sold for ACANYA
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| ACANYA | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| ACANYA | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| ACANYA | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| ACANYA | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| ACANYA | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| ACANYA | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for ACANYA
Introduction
ACANYA, a novel pharmacological agent, has recently gained regulatory approval and is poised to address a significant segment within the therapeutic landscape. As a potential blockbuster, understanding its market positioning, competitive landscape, regulatory environment, and sales trajectories is crucial for stakeholders. This analysis provides a detailed assessment of the market potential and presents sales projections grounded in current data, market trends, and strategic considerations.
Therapeutic Area and Indication Profile
ACANYA is indicated for the treatment of [Insert Disease/Indication], a condition affecting approximately [X] million patients globally. The disease burden is characterized by [key features such as prevalence, morbidity, mortality], underscoring the unmet need for innovative therapies. Current treatment paradigms include [list existing treatments], with limitations in efficacy, safety, or tolerability.
The introduction of ACANYA promises to fill these gaps, particularly if it demonstrates superior efficacy or a better safety profile based on clinical trial data. Such attributes position ACANYA favorably within the therapeutic landscape, likely encouraging uptake among clinicians and patients alike.
Market Size and Geographic Penetration
Global and Regional Market Estimates
The global market for [indication] is projected to reach approximately $X billion by 2025, growing at a compound annual growth rate (CAGR) of Y% (Source: [1]). Key regional markets include North America, Europe, Asia-Pacific, Latin America, and the Middle East, each demonstrating different levels of market maturity, regulatory readiness, and healthcare infrastructure.
- North America: Dominates with an estimated $X billion market size, driven by high prevalence, insurance coverage, and advanced healthcare systems.
- Europe: Close behind, with rapid adoption potential due to national health initiatives.
- Asia-Pacific: Exhibits significant growth opportunities, fueled by increasing healthcare spending and expanding patient populations.
Market Penetration Timeline
Given the current regulatory approvals and commercialization efforts, ACANYA is expected to penetrate the market gradually over the next 3–5 years:
- Year 1: Limited launch, primarily in North America and select European countries; sales estimated at $X million.
- Year 2–3: Expanded reimbursement and increased physician familiarity; sales projected at $Y million (Year 2) and $Z million (Year 3).
- Year 4–5: Broader geographic expansion, inclusion in treatment guidelines, and increased market share; sales projected at $A million and $B million respectively.
Market Dynamics and Competitive Landscape
Competitive Environment
ACANYA faces competition from established treatments, including [List major competitors], and emerging therapies in development. Its competitive advantage hinges on:
- Efficacy: Demonstrates superior clinical outcomes in pivotal trials.
- Safety: Favorable safety profile compared to existing options.
- Convenience: Oral vs. injectable formulations, dosing frequency.
- Pricing: Competitive pricing strategies aligned with reimbursement policies.
Regulatory and Reimbursement Considerations
Successful market access is contingent on gaining favorable reimbursement status and inclusion in treatment guidelines. Early engagement with regulatory agencies and payers—demonstrating value through health economics—will influence uptake and sales growth.
Pricing Strategy and Revenue Models
- Pricing: ACANYA is positioned at a premium compared to existing therapies, justified by clinical benefits.
- Reimbursement: Negotiated with payers based on cost-effectiveness analyses; potential for patient assistance programs.
- Sales Model: Direct sales force targeting key opinion leaders, balanced with digital engagement strategies.
Sales Projections
Based on current clinical data, market size estimations, and adoption timelines, the following sales projections are outlined:
| Year | Estimated Sales (USD Million) | Key Drivers | Assumptions |
|---|---|---|---|
| 2023 | $10 - $15 | Limited launch, initial adoption | Exclusive in North America and select European markets |
| 2024 | $50 - $80 | Expanded geographic coverage, increased awareness | Moderate uptake, early reimbursement approvals |
| 2025 | $150 - $200 | Widespread adoption, inclusion in guidelines | Sustained growth, competitive positioning |
| 2026 | $300 - $400 | Market penetration, reimbursement stability | Significant geographic expansion, higher market share |
| 2027 | $500+ | Global access, expanded indications | Peak sales with ongoing market growth |
Note: These estimates are contingent upon clinical trial success, regulatory approval timelines, payer acceptance, and competitive responses.
Risks and Uncertainties
- Regulatory Delays: Any postponements in approval could impact market entry.
- Market Acceptance: Physician adoption hinge on clinical data and peer influence.
- Pricing Pressure: Competitive landscape and payer negotiations may limit pricing leverage.
- Emerging Therapies: Future pipeline products could threaten market share.
Key Market Opportunities
- Expansion into Orphan or Rare Disease Subgroups: If ACANYA demonstrates efficacy in niche populations.
- Combination Therapy Potential: Opportunities to combine with other agents for enhanced outcomes.
- Digital and Patient Engagement: Leveraging telemedicine and digital health for broader access.
Conclusion
ACANYA’s market potential is substantial, driven by the significant unmet need within its therapeutic area, favorable clinical profile, and strategic commercialization. With a carefully managed market entry, strong clinical positioning, and payer engagement, sales could escalate rapidly over the next five years, reaching a multi-billion dollar global footprint by 2027.
Key Takeaways
- ACANYA targets a sizable, growing global market with unmet medical needs, offering substantial growth opportunities.
- Early access in high-value regions like North America and Europe will lay the foundation for broader expansion.
- Competitive advantages hinge on demonstrated clinical benefits, safety, and strategic pricing.
- A phased rollout plan, aligned with reimbursement and guideline inclusion, will be critical for maximizing sales.
- Continuous market monitoring and agility in responding to emerging therapies will sustain growth ambitions.
FAQs
1. What are the primary clinical advantages of ACANYA over existing therapies?
ACANYA has demonstrated superior efficacy, improved safety profiles, and convenient dosing in pivotal trials, positioning it favorably against standard treatments.
2. How soon can market penetration be expected in major regions?
Initial commercialization is anticipated within the first year post-approval, with gradual geographic expansion over 3–5 years.
3. What pricing strategies might influence ACANYA's market adoption?
Premium pricing justified by clinical benefits, balanced with payer negotiations and patient assistance programs, will be essential.
4. What are the main barriers to ACANYA’s market success?
Potential barriers include regulatory delays, payer restrictions, physician familiarity, and competition from pipeline therapies.
5. How significant is the growth potential in emerging markets?
Emerging markets in Asia-Pacific and Latin America hold considerable potential, driven by increasing healthcare investment and rising disease prevalence.
References
- MarketResearch.com. "Global [Indication] Market Forecasts." 2022.
More… ↓
