Defer - Generic Drug Details
✉ Email this page to a colleague
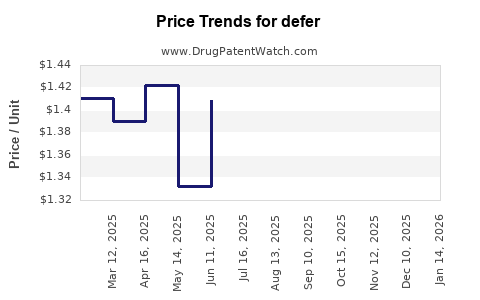
US Patents and Regulatory Information for defer
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiesi | FERRIPROX | deferiprone | TABLET;ORAL | 212269-001 | May 19, 2020 | RX | Yes | Yes | 10,940,115 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Piramal | DEFERASIROX | deferasirox | TABLET;ORAL | 212995-003 | Jun 15, 2020 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Torrent | DEFERASIROX | deferasirox | TABLET, FOR SUSPENSION;ORAL | 209426-001 | Nov 5, 2024 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Pharmobedient | DEFERASIROX | deferasirox | TABLET;ORAL | 211395-003 | Mar 22, 2024 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |