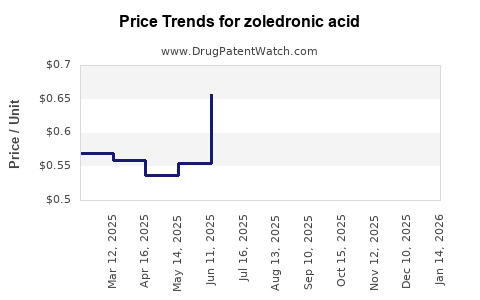
Last updated: July 27, 2025
Introduction
Zoledronic acid, a potent nitrogen-containing bisphosphonate, is widely utilized in the management of various bone disorders, mainly osteoporosis, Paget’s disease, and bone metastases associated with cancers such as breast, prostate, and multiple myeloma. Since its FDA approval in 2001, zoledronic acid has established a substantial presence within the global pharmaceutical landscape. This report delineates the market dynamics and financial trajectory influencing zoledronic acid, providing insights into factors shaping its growth, challenges, and future prospects.
Market Overview
Product Profile and Therapeutic Indications
Zoledronic acid’s primary mechanism involves inhibiting osteoclast-mediated bone resorption by disrupting the mevalonate pathway. Its efficacy in reducing skeletal-related events (SREs) in cancer patients and increasing bone mineral density (BMD) in osteoporosis has driven widespread application [1].
Initial markets centered on oncology-related indications. Over time, the expansion into osteoporosis management, especially among postmenopausal women and elderly populations, broadened its commercial footprint. Notable branded formulations include Zometa (oncology) and Reclast (osteoporosis), with generic versions entering markets post-patent expiry.
Market Size and Growth Trends
The global zoledronic acid market was valued at approximately USD 1.2 billion in 2022, with a compound annual growth rate (CAGR) projected at about 4.8% from 2023 to 2030 [2]. The escalation is driven by an aging population globally, increasing prevalence of osteoporosis, and rising cancer incidence rates.
Regionally, North America commands the largest market share due to extensive healthcare infrastructure, high awareness, and reimbursement policies. Europe follows suit, while Asia-Pacific emerges as a high-growth territory, supported by increasing healthcare access and disease prevalence.
Competitive Landscape
The market is characterized by a mix of established players—such as Novartis, Teva Pharmaceutical Industries, and Mylan—and emerging generic manufacturers. Patent expirations have catalyzed a wave of generics, significantly influencing pricing dynamics and market accessibility.
Market Dynamics
Driving Factors
- Aging Population: The demographic trend toward an older population increases osteoporosis and cancer-related skeletal complications, boosting demand for zoledronic acid [3].
- Rising Cancer Incidences: The prevalence of breast, prostate, and multiple myeloma enhances use of zoledronic acid to manage bone metastases, reducing SREs.
- Clinical Efficacy and Extended Indications: Robust clinical trials demonstrating safety and efficacy bolster clinician confidence and expand approved therapeutic uses.
- Reimbursement Policies: Favorable reimbursement frameworks in developed countries promote utilization.
Restraints and Challenges
- Safety Concerns: Rare but severe adverse effects, notably osteonecrosis of the jaw (ONJ) and atypical femur fractures, have prompted caution, influencing prescription patterns.
- Pricing Pressures and Generic Competition: Post-patent expiry, generic versions have led to substantial price reductions, squeezing profit margins for brand manufacturers.
- Alternative Therapies: The emergence of newer agents like denosumab, a monoclonal antibody inhibiting osteoclast formation, presents substitutes in several indications.
- Regulatory and Reimbursement Variability: Divergences across regions impact market access and adoption rates.
Regulatory and Policy Influences
Regulatory bodies have implemented evolving guidance on safety monitoring and reporting side effects, impacting formulary decisions. Reimbursement policies focusing on cost-effective care further influence market dynamics.
Financial Trajectory
Revenue Trends and Forecasts
Revenues from zoledronic acid have experienced a peak phase driven by branded product sales, followed by a plateau and recent decline as generic versions permeate the market. For example, Novartis’ Zometa generated peak revenues of USD 400–500 million annually before generic entrants emerged [4].
Forecasts suggest a declining trend for branded formulations, with total global revenues stabilizing around USD 800 million–USD 1 billion by 2030, largely buoyed by growth in emerging markets and new indications.
Impact of Patent Expirations
The expiration of key patents (notably in the early 2010s) opened the market to generics, reducing prices by up to 70% in some regions. This shift led to a redistribution of revenue streams from patent-holding companies to generic manufacturers.
Emerging Markets and Expansion Strategies
Pharmaceutical companies are increasingly focusing on markets in Asia-Pacific and Latin America, where growing healthcare infrastructure and increasing disease burden facilitate higher penetration. Local manufacturing, pricing strategies, and partnerships are vital to capturing these markets.
Future Outlook
Market Growth Opportunities
- Bone Health in Oncology: Expanding use in early metastatic settings with combination therapies.
- Osteoporosis in Special Populations: Addressing osteoporosis among men and premenopausal women.
- Innovative Formulations: Development of less invasive, longer-acting formulations or biosimilars to improve adherence and reduce costs.
- Real-World Evidence (RWE): Leveraging RWE to demonstrate long-term safety and cost-effectiveness can influence formulary decisions and reimbursement policies.
Challenges to Overcome
- Safety management remains critical; enhanced post-marketing surveillance is necessary.
- Competitive positioning against denosumab necessitates differentiation based on efficacy, safety profile, and cost considerations.
- Market saturation in mature economies requires diversification into new indications and markets.
Key Takeaways
- The zoledronic acid market is mature within developed countries but exhibits high growth potential in emerging regions driven by demographic and epidemiological factors.
- Patent expiries have dramatically transformed the competitive landscape, favoring generics and necessitating strategic innovation.
- Safety concerns, especially ONJ, influence prescribing patterns; ongoing surveillance and patient education are essential.
- Market expansion hinges on strategic entry into new indications, formulations, and geographic zones, particularly within oncology and osteoporosis among under-served populations.
- Collaboration with healthcare payers, physicians, and patient advocacy groups will be crucial in shaping the drug's financial trajectory moving forward.
FAQs
-
What factors primarily influence the price decline of zoledronic acid post-patent expiry?
Patent expiries open the market to generic manufacturers, significantly increasing competition. This results in substantial price reductions—sometimes up to 70%—due to intensified price competition and increased supply, making the drug more accessible and reducing revenue for original patent holders.
-
How does zoledronic acid compare to newer therapies like denosumab?
While zoledronic acid effectively manages SREs and osteoporosis, denosumab offers similar benefits with some advantages, such as less renal toxicity and administration options; however, it can be more expensive, and safety profiles differ. The choice depends on patient-specific factors and healthcare system preferences.
-
What safety concerns are associated with zoledronic acid?
The most significant risks include osteonecrosis of the jaw and atypical femur fractures. These rare but severe adverse effects necessitate monitoring, dental assessments prior to therapy, and patient education to mitigate risks.
-
What is the outlook for zoledronic acid's market growth in Asia-Pacific?
The Asia-Pacific region shows promising growth driven by rising osteoporosis and cancer rates, increasing healthcare expenditure, and greater healthcare access. Local manufacturing and tailored pricing strategies will be pivotal for market penetration.
-
Are there any ongoing developments or future innovations associated with zoledronic acid?
Researchers are exploring formulations with extended duration, biosimilars to reduce costs, and combination therapies. Additionally, better safety profiles and real-world effectiveness data could enhance market competitiveness.
References
[1] Rosenberg, J., & Mankin, H. (2014). Mechanisms of bisphosphonate action in bone. Clinical Reviews in Bone and Mineral Metabolism, 12(3), 160-171.
[2] MarketsandMarkets. (2022). Zoledronic acid market forecast.
[3] WHO. (2021). Global Ageing and Health.
[4] Novartis Annual Reports. (2010-2020). Zometa revenue data.
Note: Figures cited are approximate and based on market research reports; actual data may vary.