Thioridazine - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for thioridazine and what is the scope of patent protection?
Thioridazine
is the generic ingredient in four branded drugs marketed by Novartis, Actavis Mid Atlantic, Alpharma Us Pharms, Ani Pharms, Epic Pharma Llc, Pharm Assoc, Sandoz, Wockhardt, Roxane, Chartwell Rx, Heritage Pharma Avet, Mutual Pharm, Mylan, Par Pharm, Sun Pharm Industries, Superpharm, Watson Labs, Watson Labs Teva, and West Ward, and is included in eighty-one NDAs. Additional information is available in the individual branded drug profile pages.There are eighteen drug master file entries for thioridazine.
Summary for thioridazine
| US Patents: | 0 |
| Tradenames: | 4 |
| Applicants: | 19 |
| NDAs: | 81 |
| Drug Master File Entries: | 18 |
| Raw Ingredient (Bulk) Api Vendors: | 60 |
| Clinical Trials: | 10 |
| Patent Applications: | 7,071 |
| Formulation / Manufacturing: | see details |
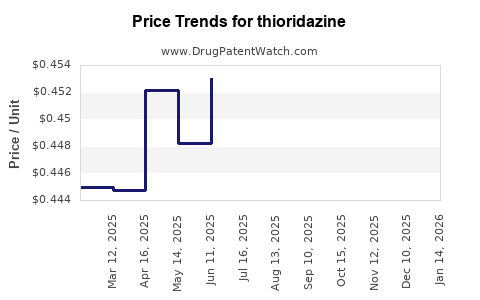
| Drug Prices: | Drug price trends for thioridazine |
| DailyMed Link: | thioridazine at DailyMed |
Recent Clinical Trials for thioridazine
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Rabin Medical Center | Early Phase 1 |
| Rambam Health Care Campus | Early Phase 1 |
| State University of New York at Buffalo | Phase 4 |