Hydrogen peroxide - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for hydrogen peroxide and what is the scope of patent protection?
Hydrogen peroxide
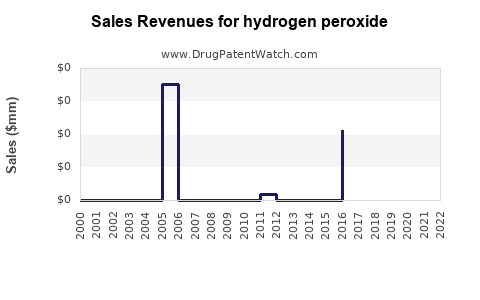
is the generic ingredient in one branded drug marketed by Aclaris and is included in one NDA. There are five patents protecting this compound. Additional information is available in the individual branded drug profile pages.Hydrogen peroxide has eighteen patent family members in sixteen countries.
There are four drug master file entries for hydrogen peroxide.
Summary for hydrogen peroxide
| International Patents: | 18 |
| US Patents: | 5 |
| Tradenames: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Drug Master File Entries: | 4 |
| Raw Ingredient (Bulk) Api Vendors: | 155 |
| Clinical Trials: | 98 |
| Patent Applications: | 5,670 |
| Formulation / Manufacturing: | see details |
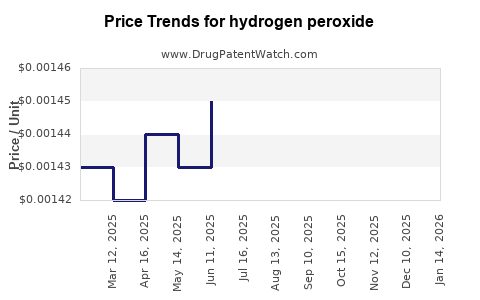
| Drug Prices: | Drug price trends for hydrogen peroxide |
| What excipients (inactive ingredients) are in hydrogen peroxide? | hydrogen peroxide excipients list |
| DailyMed Link: | hydrogen peroxide at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for hydrogen peroxide
Generic Entry Date for hydrogen peroxide*:
Constraining patent/regulatory exclusivity:
Dosage:
SOLUTION;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for hydrogen peroxide
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Synedgen, Inc. | N/A |
| Louisiana State University Health Sciences Center in New Orleans | N/A |
| University of Baghdad | Phase 3 |
Medical Subject Heading (MeSH) Categories for hydrogen peroxide
US Patents and Regulatory Information for hydrogen peroxide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for hydrogen peroxide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for hydrogen peroxide
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2015249841 | Peroxide formulations and methods and applicators for using the same | ⤷ Try a Trial |
| Portugal | 3134061 | ⤷ Try a Trial | |
| Israel | 248462 | פורמולציות פראוקסיד ושיטות ויישומים לשימוש בהן (Peroxide formulations and methods and applicators for using the same) | ⤷ Try a Trial |
| Japan | 2017513907 | 過酸化物製剤ならびにその使用のための方法および塗布器 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2015164427 | ⤷ Try a Trial | |
| Mexico | 2016013826 | FORMULACIONES DE PEROXIDO Y METODOS Y APLICACIONES PARA UTILIZARLAS. (PEROXIDE FORMULATIONS AND METHODS AND APPLICATORS FOR USING THE SAME.) | ⤷ Try a Trial |
| Canada | 2946568 | FORMULES DE PEROXYDE ET PROCEDES ET APPLICATEURS POUR LEUR UTILISATION (PEROXIDE FORMULATIONS AND METHODS AND APPLICATORS FOR USING THE SAME) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for hydrogen peroxide
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1968948 | 2021C/549 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SELUMETINIB (Y COMPRIS TOUS SELS PHARMACEUTIQUEMENT ACCEPTABLES (EN PARTICULIER HYDROGENOSULFATE), ESTERS, SOLVATES OU ENANTIOMERES DE CEUX-CI); AUTHORISATION NUMBER AND DATE: EU/1/21/1552 20210619 |
| 1419152 | CR 2012 00019 | Denmark | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRIN OG FARMACEUTISK ACCEPTABLE ADDITIONSSALTE DERAF, HERUNDER HYDROGENCHLORIDSALTET AF RILPIVIRIN; REG. NO/DATE: EU/1/11/736/001 20111128 |
| 3106463 | 2020C/507 | Belgium | ⤷ Try a Trial | PRODUCT NAME: VITRAKVI - LAROTRECTINIB ET/OU SES SELS ACCEPTABLES EN PHARMACIE, EN PARTICULIER L'HYDROGENO SULFATE DE LAROTRECTINIB; AUTHORISATION NUMBER AND DATE: EU/1/19/1385 20190923 |
| 2523731 | 2090024-7 | Sweden | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, INCLUDING OSILODROSTAT DIHYDROGEN PHOSPHATE; REG. NO/DATE: EU/1/19/1407 20200113 |
| 2523731 | CA 2020 00025 | Denmark | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, HERUNDER OSILODROSTATDIHYDROGENFOSFAT; REG. NO/DATE: EU/1/19/1407 20200113 |
| 3106463 | 2090009-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: LAROTRECTINIB,OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, PARTICULARLY LAROTRECTINIB SULFATE INCLUDING LAROTRECTINIB HYDROGEN SULFATE; REG. NO/DATE: EU/1/19/1385 20190923 |
| 2523731 | 2020012 | Norway | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT ELLER ET FARMASOEYTISK AKSEPTABELT SALT DERAV, INKLUDERT OSILODROSTAT DIHYDROGENFOSFAT; REG. NO/DATE: EU/1/19/1407 20200203 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |