Fidaxomicin - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for fidaxomicin and what is the scope of patent protection?
Fidaxomicin
is the generic ingredient in two branded drugs marketed by Cubist Pharms Llc and Actavis Labs Fl, and is included in three NDAs. There are six patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Fidaxomicin has one hundred and fifty-eight patent family members in thirty-six countries.
There are two drug master file entries for fidaxomicin. One supplier is listed for this compound.
Summary for fidaxomicin
| International Patents: | 158 |
| US Patents: | 6 |
| Tradenames: | 2 |
| Applicants: | 2 |
| NDAs: | 3 |
| Drug Master File Entries: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 61 |
| Clinical Trials: | 36 |
| Patent Applications: | 1,083 |
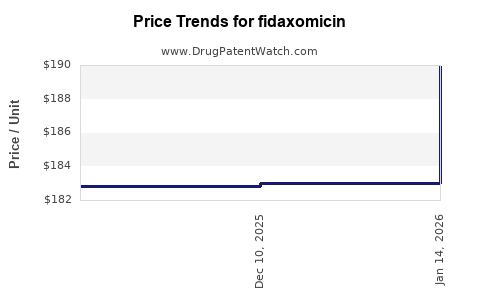
| Drug Prices: | Drug price trends for fidaxomicin |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for fidaxomicin |
| What excipients (inactive ingredients) are in fidaxomicin? | fidaxomicin excipients list |
| DailyMed Link: | fidaxomicin at DailyMed |
Recent Clinical Trials for fidaxomicin
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hospital Universitario Evangelico de Curitiba | Phase 3 |
| Benoit Guery | Phase 3 |
| Hellenic Institute for the Study of Sepsis | Phase 2 |
Pharmacology for fidaxomicin
| Drug Class | Macrolide Antibacterial |
Anatomical Therapeutic Chemical (ATC) Classes for fidaxomicin
Paragraph IV (Patent) Challenges for FIDAXOMICIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| DIFICID | Tablets | fidaxomicin | 200 mg | 201699 | 1 | 2015-05-27 |
US Patents and Regulatory Information for fidaxomicin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cubist Pharms Llc | DIFICID | fidaxomicin | TABLET;ORAL | 201699-001 | May 27, 2011 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Cubist Pharms Llc | DIFICID | fidaxomicin | TABLET;ORAL | 201699-001 | May 27, 2011 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Cubist Pharms Llc | DIFICID | fidaxomicin | TABLET;ORAL | 201699-001 | May 27, 2011 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Cubist Pharms Llc | DIFICID | fidaxomicin | FOR SUSPENSION;ORAL | 213138-001 | Jan 24, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for fidaxomicin
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Tillotts Pharma GmbH | Dificlir | fidaxomicin | EMEA/H/C/002087 Dificlir film-coated tablets is indicated for the treatment of Clostridioides difficile infections (CDI) also known as C. difficile-associated diarrhoea (CDAD) in adult and paediatric patients with a body weight of at least 12.5 kg.Consideration should be given to official guidelines on the appropriate use of antibacterial agents.Dificlir granules for oral suspension is indicated for the treatment of Clostridioides difficile infections (CDI) also known as C. difficile-associated diarrhoea (CDAD) in adults and paediatric patients from birth to < 18 years of age.Consideration should be given to official guidelines on the appropriate use of antibacterial agents. |
Authorised | no | no | no | 2011-12-05 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for fidaxomicin
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2305245 | Traitement de maladies consecutives a un traitement antibiotique (Treatment of diseases associated with the use of antibiotics) | ⤷ Sign Up |
| Poland | 2070530 | ⤷ Sign Up | |
| Mexico | 344601 | TRATAMIENTO DE ENFERMEDADES ASOCIADAS CON EL USO DE ANTIBIOTICOS. (TREATMENT OF DISEASES ASSOCIATED WITH THE USE OF ANTIBIOTICS.) | ⤷ Sign Up |
| Mexico | 2009007730 | POLIMORFOS MACROCILICOS, COMPOSICIONES QUE COMPRENDEN TALES POLIMORFOS, Y METODOS PARA SU USO Y FABRICACION. (MACROCYCLIC POLYMORPHS, COMPOSITIONS COMPRISING SUCH POLYMORPHS, AND METHODS OF USE AND MANUFACTURE THEREOF.) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for fidaxomicin
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1539977 | C 2015 015 | Romania | ⤷ Sign Up | PRODUCT NAME: FIDAXOMICIN; NATIONAL AUTHORISATION NUMBER: EU/1/11/733/001, EU/1/11/733/002, EU/1/11/733/003, EU/1/11/733/004; DATE OF NATIONAL AUTHORISATION: 20111205; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/11/733/001, EU/1/11/733/002, EU/1/11/733/003, EU/1/11/733/004; DATE OF FIRST AUTHORISATION IN EEA: 20111205 |
| 1539977 | C201530022 | Spain | ⤷ Sign Up | PRODUCT NAME: FIDAXOMICINA; NATIONAL AUTHORISATION NUMBER: EU/1/11/733/001-004; DATE OF AUTHORISATION: 20111207; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/11/733/001-004; DATE OF FIRST AUTHORISATION IN EEA: 20111207 |
| 1539977 | C20150013 00220 | Estonia | ⤷ Sign Up | PRODUCT NAME: FIDAKSOMITSIIN;REG NO/DATE: EU/1/11/733 07.12.2011 |
| 1539977 | 122015000026 | Germany | ⤷ Sign Up | PRODUCT NAME: FIDAXOMICIN; REGISTRATION NO/DATE: EU/1/11/733/001-004 20111205 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.