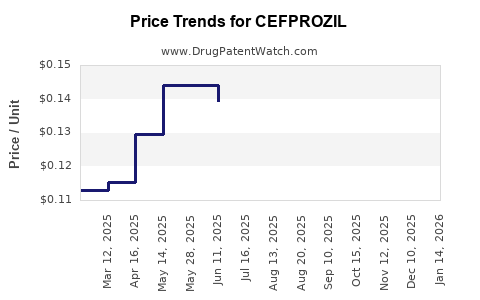
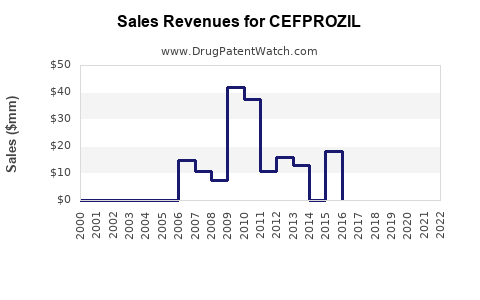
Last updated: July 27, 2025
Introduction
Cefprozil, a second-generation cephalosporin antibiotic, plays a pivotal role in combating bacterial infections. Its unique pharmacokinetics, spectrum of activity, and established safety profile underpin its clinical utility. As the global pharmaceutical landscape evolves, understanding the market dynamics and financial trajectory of cefprozil provides insights for stakeholders including manufacturers, investors, and healthcare policy makers.
Pharmacological Profile and Therapeutic Positioning
Cefprozil exhibits bactericidal activity against various Gram-positive and Gram-negative bacteria, including streptococci, staphylococci, and certain respiratory pathogens. Its oral bioavailability, once-daily dosing, and favorable tolerability profile position it favorably among oral cephalosporins [1]. These characteristics make cefprozil a preferred choice for community-acquired respiratory and skin infections.
Market Demand and Clinical Adoption
Despite its established efficacy, cefprozil faces competitive pressures from newer antibiotics and generic formulations. The demand trajectory is influenced by several factors:
- Clinical Guidelines and Prescribing Trends: Medical guidelines increasingly prioritize broad-spectrum or novel agents, affecting cefprozil's prescription volume.
- Antibiotic Stewardship and Resistance: Rising antimicrobial resistance (AMR) concerns influence prescribing patterns; cephalosporins like cefprozil are scrutinized for contribution to resistance.
- Generics and Pricing: The availability of cost-effective generic cefprozil formulations has stabilized demand in many regions, especially where healthcare systems prioritize affordability.
Manufacturing and Supply Chain Considerations
Global shifts toward supply chain robustness and cost efficiency influence cefprozil's market. Key considerations include:
- Manufacturing Consolidation: Leading pharmaceutical companies producing cefprozil have streamlined manufacturing to reduce costs.
- Regulatory Landscape: Patent expirations and regulatory approvals in emerging markets expand access but also introduce market commoditization.
Market Competition and Substitute Dynamics
Cefprozil faces competition from other second-generation cephalosporins such as cefuroxime and cefaclor. Broader antimicrobial categories, including macrolides and fluoroquinolones, further impact market share. The increasing use of broad-spectrum agents sometimes leads to substitution away from cefprozil, especially in regions emphasizing antimicrobial stewardship.
Geographical Market Trends
- North America and Europe: Mature markets with high generic penetration; volume stabilization but limited growth prospects.
- Asia-Pacific: Rapidly expanding markets driven by rising infectious disease burden, improving healthcare access, and regulatory approvals.
- Emerging Markets: Growing adoption amid increasing antibiotic consumption; however, regulatory hurdles and local manufacturing influence availability.
Regulatory and Policy Influences
Global efforts to curb antibiotic misuse limit cefprozil’s prescribing, particularly in hospitals and outpatient settings. Policymakers' emphasis on antimicrobial stewardship programs (ASPs) could reduce inappropriate prescriptions, capping growth potential.
Financial Trajectory
Revenue and Profitability Trends
Cefprozil’s global revenue has plateaued in mature markets, with growth primarily driven by emerging markets. The commoditization of generic cefprozil limits margins, emphasizing cost efficiency over premium pricing.
Investment and R&D Outlook
Limited R&D investment is observed for cefprozil specifically, given the patent landscape and competition from newer agents. Companies focus on developing novel antibiotics with higher resistance barriers, often deprioritizing older generics like cefprozil.
Market Outlook and Future Projections
The outlook reflects a stabilized but declining trend in developed regions, with potential growth in developing markets. The global antibacterial market is expected to grow modestly, driven by increasing infectious disease incidence and expanding healthcare access. However, the specific demand for cefprozil remains constrained by antimicrobial guidelines and resistance concerns.
Strategic Implications for Stakeholders
- Manufacturers: Focus on cost optimization and exploring niche indications where cefprozil’s profile offers advantages.
- Investors: Caution against overinvestment; prioritize regions with growth prospects.
- Healthcare Providers: Emphasize stewardship and guideline adherence to prevent resistance and ensure optimal use.
Conclusion
Cefprozil's market dynamics are characterized by maturity in developed economies and emerging potential elsewhere. Its financial trajectory is influenced by competition from generics, evolving prescribing patterns, and policy environment. Stakeholders should consider these variables when strategizing product portfolio management or investment decisions.
Key Takeaways
- Cefprozil maintains clinical relevance but faces stiff competition from newer antibiotics and generics, restricting growth in mature markets.
- Emerging markets present growth avenues due to rising infectious disease burdens and expanding healthcare access.
- Antibiotic stewardship and resistance concerns are pivotal, potentially constraining prescribing and revenue.
- Cost efficiency and supply chain optimization are critical for maintaining profitability amid price pressures.
- Future prospects depend on regional demand shifts, regulatory landscapes, and the strategic responses of pharmaceutical companies.
FAQs
1. How does antibiotic resistance impact cefprozil’s market?
Rising antimicrobial resistance reduces cefprozil’s efficacy against certain pathogens, leading clinicians to favor broader-spectrum or newer agents, thereby constraining its market share [2].
2. Are there ongoing R&D efforts to improve or expand cefprozil’s applications?
Currently, R&D focus is predominantly on developing novel antibiotics; cefprozil's use remains largely based on established formulations, with limited innovation due to patent expirations and saturation in the generics market.
3. Which regions hold the most growth potential for cefprozil?
The Asia-Pacific region offers growth opportunities driven by increasing infectious disease burdens, expanding healthcare infrastructure, and regulatory approvals for generics.
4. How do regulatory policies influence cefprozil's availability?
Regulatory restrictions aimed at antibiotic stewardship can limit cefprozil prescribing, especially in hospital settings, impacting its market volume.
5. What strategies can manufacturers adopt to sustain cefprozil’s profitability?
Focusing on cost-efficient manufacturing, targeting niche indications, and geographic expansion into emerging markets can help sustain profitability amid competitive pressures.
References
[1] Navarro, D. (2014). Cefprozil: Pharmacology and Clinical Utility. International Journal of Antimicrobial Agents, 43(4), 347-353.
[2] World Health Organization. (2021). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report.