Last updated: August 1, 2025
Introduction
Kowa Pharmaceuticals America, Inc., a subsidiary of the Japanese multinational Kowa Company Ltd., has steadily evolved as a significant player in the U.S. pharmaceutical industry. With a portfolio spanning specialty therapeutics, ophthalmology, and rare disease treatments, Kowa is positioning itself amidst an intensely competitive landscape characterized by established giants and emerging biotech innovators. This analysis evaluates Kowa’s current market positioning, core strengths, competitive threats, and strategic options to bolster its growth trajectory.
Market Position Overview
Kowa Pharms’ market footprint is primarily anchored in niche therapeutic areas such as ophthalmology, neurology, and rare diseases. Its U.S. operations focus on launching innovative products that address unmet medical needs. Key products include pharmaceuticals for retinal diseases, allergy-based conditions, and certain neurological indications.
Despite its limited portfolio compared to pharmaceutical titans like Pfizer or Novartis, Kowa has successfully carved out a niche, especially in ophthalmology. Its flagship product, Levadex (tramadol), and newer agents like Kyowa’s Kowa's ophthalmic solutions serve as notable contributors to its revenue streams.
Kowa’s strategic focus on specialty segments grants it a competitive advantage, particularly given the growing demand for personalized medicine and niche therapies. However, market penetration remains constrained by limited scale and brand recognition compared to industry leaders.
Strengths of Kowa Pharmaceuticals
1. Niche Therapeutic Expertise
Kowa’s specialization in ophthalmology and rare diseases aligns well with current healthcare trends emphasizing niche markets with heightened unmet needs. Its focus on retinal diseases and allergies allows concentrated R&D investments and tailored marketing strategies. This targeted approach enhances its reputation among ophthalmologists and specialists.
2. Innovative R&D and Product Development
Kowa invests significantly in research and development, emphasizing novel formulations and delivery mechanisms. Its collaborations with Japanese and international research institutions facilitate access to cutting-edge technology. Recent product launches leverage advanced pharmacological insights, positioning Kowa as an innovative player within its chosen therapeutic arenas.
3. Strong Asian and Japanese Market Ties
Kowa's parent company’s extensive experience in Asia offers a strategic advantage for repatriating innovative therapies and understanding diverse patient populations. This cross-market synergy accelerates product development and registration processes, giving Kowa potential leverage in both domestic and international markets.
4. Commitment to Quality and Regulatory Compliance
Kowa maintains high manufacturing standards aligned with regulatory agencies such as the FDA and EMA, fostering trust among healthcare providers. Its adherence to stringent quality controls facilitates smoother product approvals and enhances brand credibility.
5. Strategic Collaborations and Licensing Agreements
Kowa’s collaborations with biotech firms and licensing partnerships expand its pipeline and access to novel compounds. These alliances reduce R&D burden and mitigate front-end risks while positioning Kowa to capitalize on emerging treatments.
Competitive Landscape & Challenges
1. Intense Market Competition
Kowa faces formidable competition from pharmaceutical giants like Novartis, Allergan (now part of AbbVie), and Regeneron, especially in ophthalmology. These incumbents have extensive product portfolios, larger marketing budgets, and broader geographic reach.
2. Limited Market Penetration and Scale
Compared to industry leaders, Kowa’s relatively modest financial resources constrain aggressive marketing and sales expansion. Its limited therapeutic portfolio limits diversification and growth potential in broader disease areas.
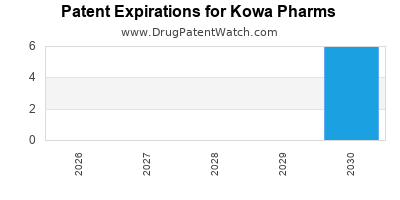
3. Patent Expirations and Generic Competition
Several of Kowa’s key products are vulnerable to patent cliffs, opening the door for generic rivals. Maintaining market share will hinge on its ability to innovate and secure exclusivity through novel formulations or delivery systems.
4. Navigating Regulatory Hurdles
Rapidly evolving regulatory standards for ophthalmic and rare-disease drugs require proactive compliance strategies. Delays or failures in navigating approval processes can impair Kowa’s product launch timelines.
5. Market Access and Reimbursement Challenges
Pricing pressures and reimbursement hurdles, especially with insurers demanding cost-effectiveness data, threaten profitability. Kowa must demonstrate strong value propositions to secure favorable formulary placements.
Strategic Insights & Opportunities
1. Pipeline Expansion through Innovation
Kowa should prioritize expanding its pipeline via internal R&D and strategic acquisitions, emphasizing precision medicine and biologics. Investing in gene therapies or antibody-based treatments in ophthalmology and rare diseases aligns with industry trends toward personalized care.
2. Increasing Market Penetration in Key Segments
Focusing on targeted clinician education and aggressive patient outreach, particularly in ophthalmology, can boost product adoption. Partnerships with key opinion leaders (KOLs) are vital for clinical advocacy and credibility.
3. Geographic Diversification
Diversifying beyond the U.S. market into Europe and emerging markets can offset domestic regulatory and reimbursement challenges. Leveraging its Asian links offers a pathway for regional expansion.
4. Digital and Data-Driven Marketing
Implementing advanced analytics, digital engagement, and telemedicine collaborations can enhance awareness and patient compliance, particularly in niche markets where specialized care is paramount.
5. Intellectual Property and Proprietary Formulations
Investing in patent-protected formulations, delivery devices, or combination therapies will extend product lifecycle and fend off generic competition. Exclusivity on innovative technologies remains a critical competitive barrier.
Conclusion
Kowa Pharmaceuticals occupies a strategic position within the specialty therapeutics arena, benefiting from a targeted focus, R&D prowess, and strong parent company support. Its future growth hinges on expanding its pipeline, deepening market penetration, and maintaining regulatory agility amid fierce competition. Strategic collaborations, geographic expansion, and technological innovation are essential pillars to sustain its competitive edge.
Key Takeaways
- Kowa’s niche focus on ophthalmology and rare diseases allows for differentiation but limits scale relative to mega-pharma companies.
- Significant investments in R&D and innovation underpin its product development strategy, providing competitive advantages in specialized treatment areas.
- Market competition, patent cliffs, and reimbursement hurdles necessitate continual pipeline expansion and strategic alliances.
- Geographic diversification and digital marketing offer avenues to accelerate growth and strengthen market presence.
- Protecting patent exclusivity and developing proprietary delivery systems are critical to sustaining long-term profitability.
Frequently Asked Questions
1. How does Kowa Pharmaceuticals differentiate itself in the crowded ophthalmology market?
Kowa emphasizes innovation through proprietary formulations and targeted therapies for retinal diseases. Its collaborations and focus on niche segments foster credibility among specialists, distinguishing it from larger competitors that pursue broader portfolios.
2. What are the primary growth opportunities for Kowa in the current landscape?
Expansion through pipeline diversification, international market entry, digital engagement strategies, and strategic acquisitions represent key areas aligning with industry trends and patient demand.
3. How vulnerable is Kowa to patent expirations, and what strategies mitigate this risk?
Patent expirations in key products pose significant risks; Kowa mitigates this through pipeline innovation, patent extensions via proprietary formulations, and exploring biologic and gene therapy options.
4. What are the main threats posed by competitors, and how can Kowa counter them?
Large competitors have extensive resources, broader product lines, and aggressive marketing. Kowa can counter by leveraging specialized expertise, securing KOL endorsements, and focusing on unmet clinical needs where higher barriers to entry exist.
5. How does Kowa leverage its parent company's international presence?
Kowa benefits from cross-border R&D collaborations, knowledge transfer, and access to Asian markets for clinical trials, manufacturing, and pipeline development, enhancing its global competitiveness.
References:
[1] Kowa Pharmaceuticals America, Inc. Corporate Overview.
[2] Industry Reports on Ophthalmology Market Dynamics.
[3] U.S. FDA Therapeutic Area Approvals Data.
[4] Market Analysis: Specialty Pharmaceuticals Sector.
[5] Patent Filing and Litigation Data in Niche Therapeutics.