Eirgen Company Profile
✉ Email this page to a colleague
What is the competitive landscape for EIRGEN, and when can generic versions of EIRGEN drugs launch?
EIRGEN has one approved drug.
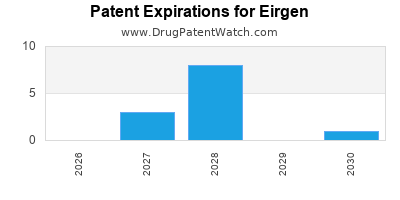
There are sixteen US patents protecting EIRGEN drugs.
There are one hundred and thirty-six patent family members on EIRGEN drugs in thirty-six countries and eleven supplementary protection certificates in seven countries.
Drugs and US Patents for Eirgen
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 8,778,373 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 10,213,442 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 9,498,486 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 11,154,509 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 10,300,078 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 8,207,149 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 9,861,644 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Eirgen
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | 6,582,727 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Eirgen Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 3888638 | ⤷ Try a Trial |
| Denmark | 2481400 | ⤷ Try a Trial |
| Germany | 202014011525 | ⤷ Try a Trial |
| Hong Kong | 1256895 | ⤷ Try a Trial |
| Portugal | 1993559 | ⤷ Try a Trial |
| South Korea | 20170085141 | ⤷ Try a Trial |
| Lithuania | 3332773 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Eirgen Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2481400 | 301085 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOL IN IEDERE VORM ZOALS BESCHERMD DOOR HET BASISOCTROOI; NATIONAL REGISTRATION NO/DATE: 124799 20200922; FIRST REGISTRATION: DE 2202115.00.00 20200819 |
| 2481400 | CA 2020 00059 | Denmark | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOL; NAT. REG. NO/DATE: 62564 20200910; FIRST REG. NO/DATE: UK PL 50784/0005-0001 20200721 |
| 2481400 | 122020000079 | Germany | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOL UND/ODER PHARMAZEUTISCH AKZEPTABLE SALZE UND HYDRATE DAVON, BEVORZUGT CALCIFEDIOLMONOHYDRAT; NAT. REGISTRATION NO/DATE: 2202115.00.00 20200818; FIRST REGISTRATION: VEREINIGTES KOENIGREICH GROSSBRITANNIEN UND NORDIRLAND PL 50784/0005 - 0001 20200721 |
| 2968172 | SPC/GB20/043 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOL, AND/OR A SALT OR HYDRATE THEREOF, IN PARTICULAR CALCIFEDIOL MONOHYDRATE; REGISTERED: UK PL 50784/0005-0001 20200721 |
| 2968172 | 132021000000074 | Italy | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOLO(RAYALDEE); AUTHORISATION NUMBER(S) AND DATE(S): 047870011, 20201201;PL 50784/0005, 20200721 |
| 2481400 | C202130022 | Spain | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOL; NATIONAL AUTHORISATION NUMBER: 85519-DE/H/5590/001/DC; DATE OF AUTHORISATION: 20201230; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): DE/H/5590/001/DC; DATE OF FIRST AUTHORISATION IN EEA: 20200721 |
| 2968172 | 122021000009 | Germany | ⤷ Try a Trial | PRODUCT NAME: CALCIFEDIOL UND/ODER PHARMAZEUTISCH AKZEPTABLE SALZE UND HYDRATE DAVON, BEVORZUGT CALCIFEDIOLMONOHYDRAT; REGISTRATION NO/DATE: 2202115.00.00 20200818 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.