Fresenius Kabi Usa Company Profile
✉ Email this page to a colleague
What is the competitive landscape for FRESENIUS KABI USA, and what generic alternatives to FRESENIUS KABI USA drugs are available?
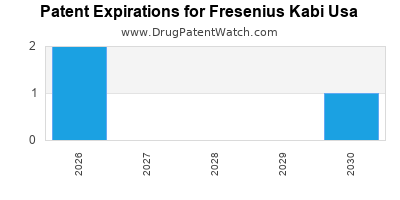
FRESENIUS KABI USA has three hundred and three approved drugs.
There are twenty-three US patents protecting FRESENIUS KABI USA drugs. There are two tentative approvals on FRESENIUS KABI USA drugs.
There are one hundred and twenty-nine patent family members on FRESENIUS KABI USA drugs in twenty-five countries and four hundred and sixty-three supplementary protection certificates in seventeen countries.
Summary for Fresenius Kabi Usa
| International Patents: | 129 |
| US Patents: | 23 |
| Tradenames: | 252 |
| Ingredients: | 186 |
| NDAs: | 303 |
| Patent Litigation for Fresenius Kabi Usa: | See patent lawsuits for Fresenius Kabi Usa |
| PTAB Cases with Fresenius Kabi Usa as petitioner: | See PTAB cases with Fresenius Kabi Usa as petitioner |
| PTAB Cases with Fresenius Kabi Usa as patent owner: | See PTAB cases with Fresenius Kabi Usa as patent owner |
Drugs and US Patents for Fresenius Kabi Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresenius Kabi Usa | CEFAZOLIN SODIUM | cefazolin sodium | INJECTABLE;INJECTION | 064169-001 | Aug 14, 1998 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Fresenius Kabi Usa | VINORELBINE TARTRATE | vinorelbine tartrate | INJECTABLE;INJECTION | 076849-001 | Apr 18, 2005 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Fresenius Kabi Usa | KANAMYCIN SULFATE | kanamycin sulfate | INJECTABLE;INJECTION | 065111-002 | Dec 17, 2002 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Fresenius Kabi Usa | NAROPIN | ropivacaine hydrochloride | SOLUTION;INJECTION | 020533-010 | Jan 4, 2011 | AP | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Fresenius Kabi Usa | XYLOCAINE 1.5% W/ DEXTROSE 7.5% | lidocaine hydrochloride | INJECTABLE;SPINAL | 016297-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Fresenius Kabi Usa | DEXMEDETOMIDINE HYDROCHLORIDE | dexmedetomidine hydrochloride | INJECTABLE;INJECTION | 208129-002 | Nov 29, 2018 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Fresenius Kabi Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Fresenius Kabi Usa | DILAUDID-HP | hydromorphone hydrochloride | INJECTABLE;INJECTION | 019034-002 | Aug 4, 1994 | 6,589,960 | ⤷ Try a Trial |
| Fresenius Kabi Usa | NAROPIN | ropivacaine hydrochloride | SOLUTION;INJECTION | 020533-001 | May 1, 1998 | 8,118,802 | ⤷ Try a Trial |
| Fresenius Kabi Usa | NAROPIN | ropivacaine hydrochloride | SOLUTION;INJECTION | 020533-006 | Sep 24, 1996 | 8,118,802 | ⤷ Try a Trial |
| Fresenius Kabi Usa | DIPRIVAN | propofol | INJECTABLE;INJECTION | 019627-002 | Jun 11, 1996 | 5,908,869*PED | ⤷ Try a Trial |
| Fresenius Kabi Usa | NAROPIN | ropivacaine hydrochloride | SOLUTION;INJECTION | 020533-005 | Sep 24, 1996 | 5,670,524 | ⤷ Try a Trial |
| Fresenius Kabi Usa | DILAUDID | hydromorphone hydrochloride | INJECTABLE;INJECTION | 019034-004 | Apr 30, 2009 | 6,589,960 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for FRESENIUS KABI USA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Injection | 2 mg/mL | ➤ Subscribe | 2011-06-22 |
| ➤ Subscribe | Injection | 10 mg/mL | ➤ Subscribe | 2011-11-04 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | Injection | 2 mg/mL, 100 mL | ➤ Subscribe | 2015-01-30 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Oral Solution | 5 mg/5mL | ➤ Subscribe | 2011-02-25 |
| ➤ Subscribe | Tablets | 2 mg, 4 mg, and 8 mg | ➤ Subscribe | 2013-08-05 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Injection | 2 mg/mL, 5 mg/mL and 10 mg/mL, 20 mL, 30 mL and 20 mL vials | ➤ Subscribe | 2006-11-13 |
| ➤ Subscribe | Injection | 2 mg/mL, 200 mL | ➤ Subscribe | 2015-09-03 |
International Patents for Fresenius Kabi Usa Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Denmark | 2376147 | ⤷ Try a Trial |
| South Korea | 100918859 | ⤷ Try a Trial |
| Australia | 2010238854 | ⤷ Try a Trial |
| Brazil | PI0408780 | ⤷ Try a Trial |
| Brazil | 0311342 | ⤷ Try a Trial |
| European Patent Office | 1673135 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Fresenius Kabi Usa Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0206283 | C980016 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVOFLOXACINE, DESGEWENST IN DE VORM VAN EEN SOLVAAT, IN HET BIJZONDER LEVOFLOXACINE-HEMIHYDRAAT; NAT. REGISTRATION NO/DATE: RVG 21810 - RVG 21812 19971209; FIRST REGISTRATION: GB 13402/0011 - 13402/0013 19970606 |
| 1441735 | 2008/010 | Ireland | ⤷ Try a Trial | PRODUCT NAME: RALTEGRAVIR OR A PHARMECEUTICALLY ACCEPTABLE SALT THEREOF, ESPECIALLY THE POTASSIUM SALT; NAT AUTHORISTION NO/DATE: EU/1/07/436/001-002 20071220; |
| 2203431 | CR 2015 00014 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150119 |
| 2377536 | 2013/024 | Ireland | ⤷ Try a Trial | PRODUCT NAME: 4-AMINOPYRIDINE (FAMPRIDINE) OR A SALT OR SOLVATE THEREOF; REGISTRATION NO/DATE: EU/1/11/699/001-EU/1/22/699-002 20110720 |
| 1732548 | C300515 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: 4-AMINOPYRIDINE EN DERIVATEN DAARVAN; REGISTRATION NO/DATE: EU/1/11/699/001-002 20110720 |
| 2340828 | PA2021502 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: SAKUBITRILAS/VALSARTANAS, KAIP SAKUBITRILO VALSARTANO NATRIO DRUSKOS KOMPLEKSAS, T.Y. (((S)-N-VALERIL-N-((2'-(1H-TETRAZOL-5-IL)-BIFENIL-4-IL)-METIL)-VALINAS)((2R,4S)-5-BIFENIL-4-IL-4-(3-KARBOKSI-PROPIONILAMINO)-2-METIL-PENTANO RUGSTIES ETILO ESTERIS)NA3; REGISTRATION NO/DATE: EU/1/15/1058 20151119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.