Generic companies with the most tentative approvals
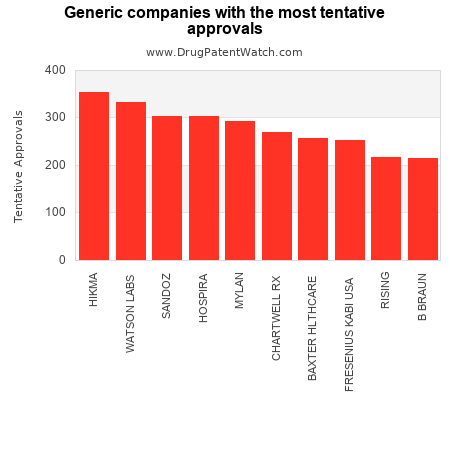
This chart shows the pharmaceutical companies with the most tentative generic drug approvals in 2023
The Food and Drug Administration (FDA) grants tentative approvals to generic entrants when their generic drug is approvable, but for the existence of unexpired patents on the branded drug. Because the FDA does not disclose the identify of Paragraph IV challengers, tentative approvals can be used to identify potential Paragraph IV filers — the patent challengers will likely have tentative approvals enabling them to rapidly launch their generic versions.
The companies with the most tentative approvals are:


