kerendia Drug Patent Profile
✉ Email this page to a colleague
When do Kerendia patents expire, and when can generic versions of Kerendia launch?
Kerendia is a drug marketed by Bayer Hlthcare and is included in one NDA. There are two patents protecting this drug.
This drug has ninety-three patent family members in forty-seven countries.
The generic ingredient in KERENDIA is finerenone. One supplier is listed for this compound. Additional details are available on the finerenone profile page.
DrugPatentWatch® Generic Entry Outlook for Kerendia
Kerendia will be eligible for patent challenges on July 9, 2025. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 12, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for kerendia?
- What are the global sales for kerendia?
- What is Average Wholesale Price for kerendia?
Summary for kerendia
| International Patents: | 93 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 33 |
| Clinical Trials: | 5 |
| Patent Applications: | 103 |
| Drug Prices: | Drug price information for kerendia |
| What excipients (inactive ingredients) are in kerendia? | kerendia excipients list |
| DailyMed Link: | kerendia at DailyMed |
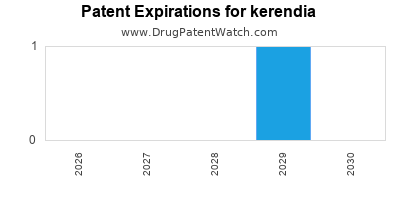
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for kerendia
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for kerendia
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Medical Center Groningen | Phase 4 |
| Boehringer Ingelheim | Phase 4 |
| University of North Carolina, Chapel Hill | Phase 2 |
Pharmacology for kerendia
| Drug Class | Nonsteroidal Mineralocorticoid-Receptor Antagonist |
| Mechanism of Action | Mineralocorticoid Receptor Antagonists |
US Patents and Regulatory Information for kerendia
kerendia is protected by two US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of kerendia is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting kerendia
Substituted-4-aryl-1,4-dihydro-1,6-naphthyridinamides and use thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting kerendia
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
INFORMATION ADDED TO THE LABELING TO DESCRIBE THE RESULTS OF FIGARO-DKD STUDY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-001 | Jul 9, 2021 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-002 | Jul 9, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-001 | Jul 9, 2021 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-002 | Jul 9, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-001 | Jul 9, 2021 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-001 | Jul 9, 2021 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-002 | Jul 9, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for kerendia
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bayer AG | Kerendia | finerenone | EMEA/H/C/005200 Kerendia is indicated for the treatment of chronic kidney disease (stage 3 and 4 with albuminuria) associated with type 2 diabetes in adults. |
Authorised | no | no | no | 2022-02-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for kerendia
When does loss-of-exclusivity occur for kerendia?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5463
Patent: AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS, SU USO EN LA FABRICACION DE UN MEDICAMENTO PARA EL TRATAMIENTO DE ENFERMEDADES CARDIOVASCULARES Y UN METODO PARA SU PREPARACION.
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 08221071
Patent: Substituted 4-aryl-1,4-dihydro-1,6-naphthyridinamides and use thereof
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0808098
Patent: 4-ARIL-1,4-DI-HIDRO-1,6-NAFTIRIDINAMIDAS SUBSTITUÍDAS E SEU USO
Estimated Expiration: ⤷ Sign Up
Patent: 2020008544
Patent: uso de 4-aril-1,4-di-hidro-1,6-naftiridinamidas substituídas, e medicamento
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 79232
Patent: AMIDES DE 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINE SUBSTITUES ETUTILISATION DE CEUX-CI (SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND USE THEREOF)
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 08000502
Patent: COMPUESTOS DERIVADOS DE AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS; PROCEDIMIENTO DE PREPARACION; MEDICAMENTO; Y USO DEL COMPUESTO PARA EL TRATAMIENTO Y/O PROFILAXIS DE ALDOSTERONISMO, PRESION SANGUINEA ALTA, FALLO CARDIACO CRONICO, SEC
Estimated Expiration: ⤷ Sign Up
China
Patent: 1641352
Patent: Substituted 4-aryl-1,4-dihydro-1,6-naphthyridinamides and use thereof
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 20951
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 976
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0150702
Estimated Expiration: ⤷ Sign Up
Cuba
Patent: 874
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Patent: 090148
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 16455
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 32206
Estimated Expiration: ⤷ Sign Up
Dominican Republic
Patent: 009000205
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 099581
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 32206
Patent: AMIDES DE 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINE SUBSTITUÉS ET UTILISATION DE CEUX-CI (SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND USE THEREOF)
Estimated Expiration: ⤷ Sign Up
France
Patent: C1017
Estimated Expiration: ⤷ Sign Up
Germany
Patent: 2007009494
Patent: Substituierte 4-Aryl-1, 4-dihydro-1,6-naphthyridinamide und ihre Verwendung (New 1,6-naphthyridine or 8-azaquinazoline derivatives useful for treating aldosteronism, hypertension, cardiac insufficiency, myocardial infarct sequelae, liver cirrhosis, renal insufficiency and stroke)
Estimated Expiration: ⤷ Sign Up
Guatemala
Patent: 0900230
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y USO
Estimated Expiration: ⤷ Sign Up
Honduras
Patent: 09001597
Patent: 4-ARIL-1, 4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 40194
Patent: 取代的 -芳基- -二氫- -萘啶酰胺和其用途 (SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND USE THEREOF 4--14--16-)
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 26441
Estimated Expiration: ⤷ Sign Up
Patent: 200015
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 0060
Patent: 4-אריל-1,4-דיהידרו-1,6-נפתירידינאמידים מותמרים ושימוש בהם (Substituted 4-aryl-1,4-dihydro-1,6-naphthyridinamides and use thereof)
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 67586
Estimated Expiration: ⤷ Sign Up
Patent: 52754
Estimated Expiration: ⤷ Sign Up
Patent: 10519232
Estimated Expiration: ⤷ Sign Up
Patent: 14012678
Patent: SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINE AMIDE AND USE THEREOF
Estimated Expiration: ⤷ Sign Up
Jordan
Patent: 18
Patent: مُركبات 4- أريل- 1 , 4 - ثنائي هيدرو -1 , 6- نافثيريدين أميد amides مُستبدلة وإستعمالها (Substituted 4-aryl-1,4-dihydro-1,6-naphthyridine amides and their use)
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 2022512
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 0748
Patent: SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND THEIR USE
Estimated Expiration: ⤷ Sign Up
Patent: 6873
Patent: SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINE AMIDES AND THEIR USE
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 09008701
Patent: 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINAMIDAS SUSTITUIDAS Y SU USO. (CARBON BLACK PELLETS AND METHOD OF FORMING SAME.)
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 245
Patent: الاميدات 4 أريل-1 ،4-ثنائي هيدرو-1،6-نافتيريدين المبدل وإستعمالها.
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 1192
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 9230
Patent: Substituted 4-aryl-1,4-dihydro-1,6-naphthyridinamides and use thereof
Estimated Expiration: ⤷ Sign Up
Panama
Patent: 70101
Patent: AMIDAS DE 4-ARIL-1,4-DIHIDRO-1, 6-NAFTIRIDINA SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 090724
Patent: AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 32206
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 32206
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 70932
Patent: ЗАМЕЩЕННЫЕ 4-АРИЛ-1,4-ДИГИДРО-1,6-НАФТИРИДИНАМИДЫ И ИХ ПРИМЕНЕНИЕ (SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINE AMIDES AND USE THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 09135659
Patent: ЗАМЕЩЕННЫЕ 4-АРИЛ-1,4-ДИГИДРО-1,6-НАФТИРИДИНАМИДЫ И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Sign Up
Saudi Arabia
Patent: 290071
Patent: مُركبات 4- أريل-4،1- ثنائي هيدرو-6،1- نافثيريدين أميدات مُستبدلة واستعمالها (Substituted 4-aryl-1,4-dihydro-1,6-naphthyridine amides and their use)
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 32206
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0905730
Patent: Substituted 4-Aryl-1,4-Dihydro-1,6-Naphthyridinamides and use thereof
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1614164
Estimated Expiration: ⤷ Sign Up
Patent: 090129992
Patent: SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND USE THEREOF
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 40803
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 15608
Estimated Expiration: ⤷ Sign Up
Patent: 74821
Estimated Expiration: ⤷ Sign Up
Patent: 0843755
Patent: Substituted 4-aryl-1, 4-dihydro-1, 6-naphthyridine amides and their use
Estimated Expiration: ⤷ Sign Up
Patent: 1340968
Patent: Substituted 4-aryl-1,4-dihydro-1,6-naphthyridine amides and their use
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 09000318
Patent: SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND USE THEREOF
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 2065
Patent: ЗАМІЩЕНІ 4-АРИЛ-1,4-ДИГІДРО-1,6-НАФТИРИДИНАМІДИ І ЇХ ЗАСТОСУВАННЯ[ЗАМЕЩЕННЫЕ 4-АРИЛ-1,4-ДИГИДРО-1,6-НАФТИРИДИНАМИДЫ И ИХ ПРИМЕНЕНИЕ (SUBSTITUTED 4-ARYL-1,4-DIHYDRO-1,6-NAPHTHYRIDINAMIDES AND USE THEREOF)
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 931
Patent: AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS Y SU USO
Estimated Expiration: ⤷ Sign Up
Patent: 952
Patent: AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS Y COMPOSICIONES FARMACÉU-TICAS QUE LAS CONTIENEN
Estimated Expiration: ⤷ Sign Up
Patent: 953
Patent: AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS Y COMPOSICIONES FARMACÉU-TICAS QUE LAS CONTIENEN
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering kerendia around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Uruguay | 36864 | PROCEDIMIENTO PARA LA PREPARACIÓN DE (4S)-4-(4-CIANO-2-METOXIFENILO)-5-ETOXI-2,8-DIMETILO-1,4-DIHIDRO-1,6-NAFTIRIDINA-3-CARBOXAMIDA Y SU PURIFICACIÓN PARA SU USO COMO PRINCIPIO ACTIVO FARMACÉUTICO | ⤷ Sign Up |
| Chile | 2008000502 | COMPUESTOS DERIVADOS DE AMIDAS DE 4-ARIL-1,4-DIHIDRO-1,6-NAFTIRIDINA SUSTITUIDAS; PROCEDIMIENTO DE PREPARACION; MEDICAMENTO; Y USO DEL COMPUESTO PARA EL TRATAMIENTO Y/O PROFILAXIS DE ALDOSTERONISMO, PRESION SANGUINEA ALTA, FALLO CARDIACO CRONICO, SEC | ⤷ Sign Up |
| Poland | 2132206 | ⤷ Sign Up | |
| Russian Federation | 2009135659 | ЗАМЕЩЕННЫЕ 4-АРИЛ-1,4-ДИГИДРО-1,6-НАФТИРИДИНАМИДЫ И ИХ ПРИМЕНЕНИЕ | ⤷ Sign Up |
| Cyprus | 1116455 | ⤷ Sign Up | |
| Lithuania | PA2022512 | ⤷ Sign Up | |
| Hungary | E044574 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for kerendia
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2132206 | C202230028 | Spain | ⤷ Sign Up | PRODUCT NAME: FINERENONA Y SUS SALES, SOLVATOS Y SOLVATOS DE LAS SALES; NATIONAL AUTHORISATION NUMBER: EU/1/21/1616; DATE OF AUTHORISATION: 20220216; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/21/1616; DATE OF FIRST AUTHORISATION IN EEA: 20220216 |
| 2132206 | CR 2022 00025 | Denmark | ⤷ Sign Up | PRODUCT NAME: FINERENONE OG DETS SALTE, SOLVATER OG SOLVATER AF SALTENE DERAF; REG. NO/DATE: EU/1/21/1616 20220217 |
| 2132206 | C02132206/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: FINERENON; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 68130 26.11.2021 |
| 2132206 | CA 2022 00025 | Denmark | ⤷ Sign Up | PRODUCT NAME: FINERENONE OG DETS SALTE, SOLVATER OG SOLVATER AF SALTENE DERAF; REG. NO/DATE: EU/1/21/1616 20220217 |
| 2132206 | 122022000030 | Germany | ⤷ Sign Up | PRODUCT NAME: FINERENON, SOWIE DESSEN SALZE, SOLVATE UND SOLVATE DER SALZE; REGISTRATION NO/DATE: EU/1/21/1616 20220216 |
| 2132206 | PA2022512 | Lithuania | ⤷ Sign Up | PRODUCT NAME: FINERENONAS; REGISTRATION NO/DATE: EU/1/21/1616 20220216 |
| 2132206 | 22C1017 | France | ⤷ Sign Up | PRODUCT NAME: FINERENONE ET SES SELS, SOLVATES ET SOLVATES DES SELS.; REGISTRATION NO/DATE: EU/1/21/1616 20220217 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


