RELISTOR Drug Patent Profile
✉ Email this page to a colleague
When do Relistor patents expire, and when can generic versions of Relistor launch?
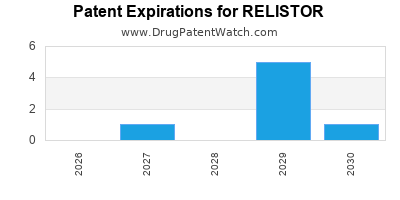
Relistor is a drug marketed by Salix Pharms and Salix and is included in two NDAs. There are twelve patents protecting this drug and three Paragraph IV challenges.
This drug has one hundred and twenty-three patent family members in thirty-six countries.
The generic ingredient in RELISTOR is methylnaltrexone bromide. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the methylnaltrexone bromide profile page.
DrugPatentWatch® Generic Entry Outlook for Relistor
Relistor was eligible for patent challenges on April 24, 2012.
There have been thirteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (methylnaltrexone bromide), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for RELISTOR?
- What are the global sales for RELISTOR?
- What is Average Wholesale Price for RELISTOR?
Summary for RELISTOR
| International Patents: | 123 |
| US Patents: | 12 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 36 |
| Clinical Trials: | 18 |
| Patent Applications: | 269 |
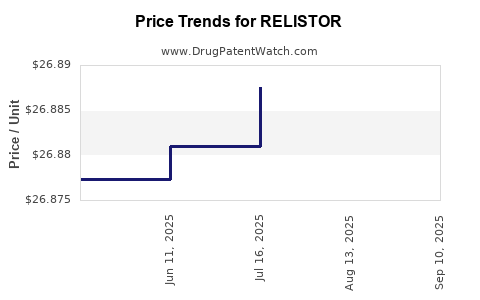
| Drug Prices: | Drug price information for RELISTOR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for RELISTOR |
| What excipients (inactive ingredients) are in RELISTOR? | RELISTOR excipients list |
| DailyMed Link: | RELISTOR at DailyMed |
Recent Clinical Trials for RELISTOR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Alabama at Birmingham | N/A |
| Odense University Hospital | Phase 2/Phase 3 |
| Asbjørn Mohr Drewes | Phase 2/Phase 3 |
Pharmacology for RELISTOR
| Drug Class | Opioid Antagonist |
| Mechanism of Action | Opioid Antagonists |
Paragraph IV (Patent) Challenges for RELISTOR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RELISTOR | Tablets | methylnaltrexone bromide | 150 mg | 208271 | 1 | 2016-09-06 |
| RELISTOR | Injection | methylnaltrexone bromide | 8 mg/0.4 mL, Single Dose Prefilled Syringe | 021964 | 1 | 2015-09-08 |
| RELISTOR | Injection | methylnaltrexone bromide | 12 mg/0.6 mL, Single Dose Vial | 021964 | 1 | 2015-07-22 |
US Patents and Regulatory Information for RELISTOR
RELISTOR is protected by thirteen US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix | RELISTOR | methylnaltrexone bromide | TABLET;ORAL | 208271-001 | Jul 19, 2016 | RX | Yes | Yes | 10,376,505 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix | RELISTOR | methylnaltrexone bromide | TABLET;ORAL | 208271-001 | Jul 19, 2016 | RX | Yes | Yes | 8,524,276 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-002 | Sep 27, 2010 | RX | Yes | Yes | 8,420,663 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-003 | Sep 27, 2010 | RX | Yes | Yes | 8,247,425 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Salix | RELISTOR | methylnaltrexone bromide | TABLET;ORAL | 208271-001 | Jul 19, 2016 | RX | Yes | Yes | 9,724,343 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-002 | Sep 27, 2010 | RX | Yes | Yes | 9,492,445 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-001 | Apr 24, 2008 | RX | Yes | Yes | 12,303,592 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RELISTOR
EU/EMA Drug Approvals for RELISTOR
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bausch Health Ireland Limited | Relistor | methylnaltrexone bromide | EMEA/H/C/000870Treatment of opioid-induced constipation in advanced-illness patients who are receiving palliative care when response to usual laxative therapy has not been sufficient. | Authorised | no | no | no | 2008-07-01 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for RELISTOR
When does loss-of-exclusivity occur for RELISTOR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3520
Patent: ANTAGONISTAS DE RECEPTORES OPIOIDES PERIFERICOS Y USOS DE LOS MISMOS
Estimated Expiration: ⤷ Get Started Free
Patent: 0491
Patent: FORMULACIONES ORALES Y SALES LIPOFILICAS DE METILNALTREXONA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09298500
Patent: Peripheral opioid receptor antagonists and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 11224275
Patent: Oral formulations and lipophilic salts of methylnaltrexone
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0919539
Patent: antagonistas de receptor de opioide periférico e empregos dos mesmos
Estimated Expiration: ⤷ Get Started Free
Patent: 2012022873
Patent: formulações orais e sais lipofílicos de metilnaltrexona
Estimated Expiration: ⤷ Get Started Free
Patent: 2020013665
Patent: seringas pré-carregadas compreendendo composição líquida de metilnaltrexona com baixo teor de tungstênio e os usos da dita composição na preparação de seringas pré-carregadas, medicamentos e kits para o tratamento de constipação induzida por opioides
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 76881
Patent: ANTAGONISTES DE RECEPTEURS OPIOIDES PERIPHERIQUES, ET LEURS UTILISATIONS (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 89798
Patent: FORMULATIONS ORALES ET SELS LIPOPHILES DE METHYLNALTREXONE (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12002192
Patent: Sal de metilnaltrexona cuyo anión es un excipiente anfifílico; composición farmacéutica para administración oral que comprende esta sal y un excipiente anfifílico; método de preparación de la composición farmaceutica; método para reducir efectos secundarios de la terapia opioide; producto; y paquete multidía.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2307874
Patent: Peripheral opioid receptor antagonists and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 2918039
Patent: Oral formulations and lipophilic salts of methylnaltrexone
Estimated Expiration: ⤷ Get Started Free
Patent: 3833634
Patent: Peripheral opioid receptor antagonists and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 5399673
Patent: Peripheral Opioid Receptor Antagonists And Uses Thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 7308125
Patent: 甲基纳曲酮的口服制剂和亲脂盐 (Oral formulations and lipophilic salts of methylnaltrexone)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 30134
Patent: Formulación orales y sales lipofílicas de metilnaltrexona
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 120476
Patent: FORMULACIONES ORALES Y SALES LIPOFÍLICAS DE METILNALTREXONA
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 12012208
Patent: FORMULACIONES ORALES Y SALES LIPOFÍLICAS DE METILNALTREXONA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 9096
Patent: ТВЕРДАЯ ЛЕКАРСТВЕННАЯ ФОРМА ДЛЯ ПЕРОРАЛЬНОГО ВВЕДЕНИЯ, СОДЕРЖАЩАЯ МЕТИЛНАЛТРЕКСОН (SOLID DOSAGE FORM FOR ORAL ADMINISTRATION COMPRISING METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1270741
Patent: ПЕРОРАЛЬНЫЕ КОМПОЗИЦИИ И ЛИПОФИЛЬНЫЕ СОЛИ МЕТИЛНАЛТРЕКСОНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 56119
Patent: ANTAGONISTES DE RÉCEPTEURS OPIOÏDES PÉRIPHÉRIQUES ET LEURS UTILISATIONS (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 71357
Patent: Formulations orales et sels lipophiles de la méthylnaltrexone (Oral formulations and lipophilic salts of methylnaltrexone)
Estimated Expiration: ⤷ Get Started Free
Patent: 86997
Patent: Antagonistes de récepteur opioïde périphérique et leurs utilisations (Peripheral opioid receptor antagonists and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Patent: 78472
Patent: FORMULATIONS ORALES DE LA MÉTHYLNALTREXONE (ORAL FORMULATIONS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 08322
Patent: SERINGUE CONTENANT DU BROMURE DE NMTX (SYRINGE CONTAINING NMTX BROMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 71151
Patent: SERINGUE CONTENANT DU BROMURE DE NMTX (SYRINGE CONTAINING NMTX BROMIDE)
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 01606550
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1200247
Patent: FORMULACIONES ORALES Y SALES LIPOFÍLICAS DE METILNALTREXONA
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 99442
Patent: 周邊的鴉片受體拮抗藥及其用途 (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 45673
Patent: 甲基納曲酮的口服製劑和親脂鹽 (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 33133
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1865
Patent: אנטאגוניסטים לקולטן חיצוני של אופיואיד ושימושיהם (Peripheral opioid receptor antagonists and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Patent: 1452
Patent: הרכבים למתן דרך הפה ומלחים ליפופילים של מתילנלטרקסון (Oral formulations and lipophilic salts of methylnaltrexone)
Estimated Expiration: ⤷ Get Started Free
Patent: 9507
Patent: אנטאגוניסטים לקולטן חיצוני של אופיואיד ושימושיהם (Peripheral opioid receptor antagonists and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 19773
Estimated Expiration: ⤷ Get Started Free
Patent: 43409
Estimated Expiration: ⤷ Get Started Free
Patent: 29955
Estimated Expiration: ⤷ Get Started Free
Patent: 47368
Estimated Expiration: ⤷ Get Started Free
Patent: 47713
Estimated Expiration: ⤷ Get Started Free
Patent: 11190259
Patent: ORAL FORMULATION AND LIPOPHILIC SALT OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Patent: 12504635
Estimated Expiration: ⤷ Get Started Free
Patent: 15129144
Patent: 末梢オピオイド受容体アンタゴニストおよびその使用 (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 16029054
Patent: メチルナルトレキソンの経口製剤および親油性塩 (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 17101053
Patent: 末梢オピオイド受容体アンタゴニストおよびその使用 (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 17206553
Patent: メチルナルトレキソンの経口製剤および親油性塩 (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 19031536
Patent: 末梢オピオイド受容体アンタゴニストおよびその使用 (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 19034958
Patent: メチルナルトレキソンの経口製剤および親油性塩 (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 20196733
Patent: 末梢オピオイド受容体アンタゴニストおよびその使用 (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0727
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9145
Patent: FORMULACIONES ORALES Y SALES LIPOFILICAS DE METILNALTREXONA. (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 8805
Patent: FORMULACIONES ORALES Y SALES LIPOFÍLICAS EN METILNALTREXONA. (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11003400
Patent: ANTAGONISTAS DE RECEPTORES DE OPIACEOS PERIFERICOS Y USO DE LOS MISMOS. (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 12009125
Patent: FORMULACIONES ORALES Y SALES LIPOFILICAS DE METILNALTREXONA. (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 18015087
Patent: ANTAGONISTAS DE RECEPTORES DE OPIACEOS PERIFERICOS Y USOS DE LOS MISMOS. (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 146
Patent: FORMULATIONS ORALLES ET SELS LIPOPHILES DE METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1859
Patent: PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 1595
Patent: Oral formulations and lipophilic salts of methylnaltrexone
Estimated Expiration: ⤷ Get Started Free
Patent: 2667
Patent: Peripheral opioid receptor antagonists and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 9972
Patent: Peripheral opioid receptor antagonists and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 2826
Patent: Oral formulations and lipophilic salts of methylnaltrexone
Estimated Expiration: ⤷ Get Started Free
Patent: 3564
Patent: Oral formulations and lipophilic salts of methylnaltrexone
Estimated Expiration: ⤷ Get Started Free
Patent: 0085
Patent: Peripheral opioid receptor antagonists and uses thereof
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 130063
Patent: FORMULACIONES ORALES Y SALES LIPOFILICAS DE METILNALTREXONA
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012501622
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 71357
Estimated Expiration: ⤷ Get Started Free
Patent: 78472
Estimated Expiration: ⤷ Get Started Free
Patent: 08322
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 08322
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 11117335
Patent: АНГОНИСТЫ ПЕРИФЕРИЧЕСКИХ РЕЦЕПТОРОВ ОПИОИДОВ И ИХ ПРИМЕНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 13157119
Patent: АНТАГОНИСТЫ ПЕРИФЕРИЧЕСКИХ РЕЦЕПТОРОВ ОПИОИДОВ И ИХ ПРИМЕНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3133
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Patent: 4393
Patent: PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 201501821R
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Patent: 201606618P
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1808498
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1566675
Estimated Expiration: ⤷ Get Started Free
Patent: 1913102
Estimated Expiration: ⤷ Get Started Free
Patent: 1982482
Estimated Expiration: ⤷ Get Started Free
Patent: 110060967
Patent: PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 130010900
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Patent: 140091071
Patent: PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 180118260
Patent: 메틸날트렉손의 경구 제형 및 친유성 염 (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 23926
Estimated Expiration: ⤷ Get Started Free
Patent: 14884
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 89293
Estimated Expiration: ⤷ Get Started Free
Patent: 05814
Estimated Expiration: ⤷ Get Started Free
Patent: 1141479
Patent: Oral formulations and lipophilic salts of methylnaltrexone
Estimated Expiration: ⤷ Get Started Free
Patent: 1622724
Patent: Oral formulations and lipophilic salts of METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 12000392
Patent: ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1717
Patent: СКЛАД ДЛЯ ПЕРОРАЛЬНОГО ВВЕДЕННЯ І ЛІПОФІЛЬНІ СОЛІ МЕТИЛНАЛТРЕКСОНУ
Estimated Expiration: ⤷ Get Started Free
Patent: 3856
Patent: ФАРМАЦЕВТИЧНІ КОМПОЗИЦІЇ МЕТИЛНАЛТРЕКСОНУ ТА НАТРІЮ ДОДЕЦИЛСУЛЬФАТУ ДЛЯ ПЕРОРАЛЬНОГО ВВЕДЕННЯ
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering RELISTOR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Ecuador | SP088752 | ⤷ Get Started Free | |
| Russian Federation | 2539387 | СОСТАВЫ ДЛЯ ПАРЕНТЕРАЛЬНОЙ ДОСТАВКИ СОЕДИНЕНИЙ И ИХ ПРИМЕНЕНИЕ (FORMULATIONS FOR PARENTERAL DELIVERY OF COMPOUNDS AND USING THEM) | ⤷ Get Started Free |
| Poland | 3178472 | ⤷ Get Started Free | |
| Japan | 2017206553 | メチルナルトレキソンの経口製剤および親油性塩 (ORAL FORMULATIONS AND LIPOPHILIC SALTS OF METHYLNALTREXONE) | ⤷ Get Started Free |
| Japan | 2017101053 | 末梢オピオイド受容体アンタゴニストおよびその使用 (PERIPHERAL OPIOID RECEPTOR ANTAGONISTS AND USES THEREOF) | ⤷ Get Started Free |
| Poland | 2368553 | ⤷ Get Started Free | |
| China | 107308125 | 甲基纳曲酮的口服制剂和亲脂盐 (Oral formulations and lipophilic salts of methylnaltrexone) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for RELISTOR
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
