drospirenone Drug Patent Profile
✉ Email this page to a colleague
When do Drospirenone patents expire, and when can generic versions of Drospirenone launch?
Drospirenone is a drug marketed by Exeltis Usa Inc, Barr, Glenmark Pharms Ltd, Hetero Labs, Hlthcare, Jubilant Cadista, Mylan Labs Ltd, Watson Labs, Apotex, Dr Reddys Labs Sa, Lupin Ltd, Naari Pte Ltd, and Watson Labs Inc. and is included in twenty NDAs. There are thirteen patents protecting this drug.
This drug has sixty-five patent family members in twenty-nine countries.
The generic ingredient in DROSPIRENONE is drospirenone; ethinyl estradiol; levomefolate calcium. There are eleven drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the drospirenone; ethinyl estradiol; levomefolate calcium profile page.
DrugPatentWatch® Generic Entry Outlook for Drospirenone
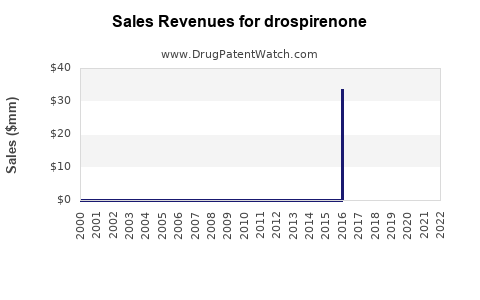
Annual sales in 2021 were $29mm indicating the motivation for generic entry (peak sales were $59mm in 2019).
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for drospirenone
| International Patents: | 65 |
| US Patents: | 13 |
| Applicants: | 13 |
| NDAs: | 20 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 98 |
| Patent Applications: | 2,065 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for drospirenone |
| Drug Sales Revenues: | Drug sales revenues for drospirenone |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for drospirenone |
| DailyMed Link: | drospirenone at DailyMed |
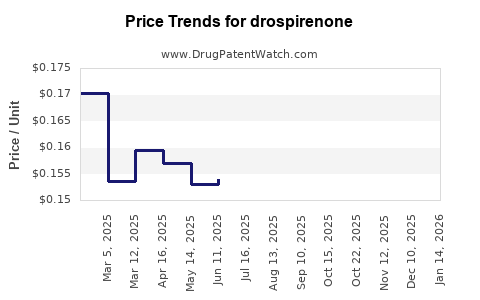
See drug prices for drospirenone
Recent Clinical Trials for drospirenone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Colorado, Denver | Phase 2 |
| Bristol Myers Squibb Company (BMS) | Phase 1 |
| Janssen Pharmaceutica N.V., Belgium | Phase 1 |
Anatomical Therapeutic Chemical (ATC) Classes for drospirenone
Paragraph IV (Patent) Challenges for DROSPIRENONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SLYND | Tablets | drospirenone | 4 mg | 211367 | 1 | 2022-01-07 |
US Patents and Regulatory Information for drospirenone
drospirenone is protected by thirteen US patents and one FDA Regulatory Exclusivity.
Patents protecting drospirenone
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY IN FEMALES OF REPRODUCTIVE AGE
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY IN FEMALES OF REPRODUCTIVE AGE
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY IN FEMALES OF REPRODUCTIVE AGE
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY IN FEMALES OF REPRODUCTIVE AGE
Synthetic progestogens and pharmaceutical compositions comprising the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY IN FEMALES OF REPRODUCTIVE AGE
FDA Regulatory Exclusivity protecting drospirenone
NEW PRODUCT
Exclusivity Expiration: ⤷ Try a Trial
International Patents for drospirenone
See the table below for patents covering drospirenone around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Peru | 20161410 | COMPOSICION FARMACEUTICA QUE COMPRENDE DROSPIRENONA Y KIT ANTICONCEPTIVO | ⤷ Try a Trial |
| South Korea | 102539030 | ⤷ Try a Trial | |
| Serbia | 63027 | FARMACEUTSKA KOMPOZICIJA KOJA OBUHVATA DROSPIRENON ZA UPOTREBU KAO KONTRACEPTIV (PHARMACEUTICAL COMPOSITION COMPRISING DROSPIRENONE FOR USE AS A CONTRACEPTIVE) | ⤷ Try a Trial |
| European Patent Office | 3632448 | COMPOSITION PHARMACEUTIQUE COMPRENANT DE LA DROSPIRÉNONE ET ENSEMBLE CONTRACEPTIF (PHARMACEUTICAL COMPOSITION COMPRISING DROSPIRENONE AND CONTRACEPTIVE KIT) | ⤷ Try a Trial |
| South Korea | 20130048227 | PHARMACEUTICAL COMPOSITION COMPRISING DROSPIRENONE AND CONTRACEPTIVE KIT | ⤷ Try a Trial |
| Slovenia | 3632448 | ⤷ Try a Trial | |
| Croatia | P20220332 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for drospirenone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0398460 | SPC/GB04/032 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL, OPTIONALLY IN THE FORM OF A HYDRATE, TOGETHER WITH DROSPIRENONE; REGISTERED: NL RVG 27505 20021211; UK PL 00053/0341 20040310 |
| 2588114 | 122021000065 | Germany | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; NAT. REGISTRATION NO/DATE: 7002248.00.00 20210426; FIRST REGISTRATION: DK 61678 20191016 |
| 3632448 | C202230031 | Spain | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONA; NATIONAL AUTHORISATION NUMBER: 84603-SE/H/1869/001/DC; DATE OF AUTHORISATION: 20191025; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SE/H/1809/001/DC; DATE OF FIRST AUTHORISATION IN EEA: 20191016 |
| 3632448 | 301186 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SLINDA; NATIONAL REGISTRATION NO/DATE: RVG 127386 20210317; FIRST REGISTRATION: DK 61678 20191022 |
| 3632448 | CA 2022 00016 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; NAT. REG. NO/DATE: 61678 20191016; FIRST REG. NO/DATE: DK 61678 20191016 |
| 3632448 | 122022000040 | Germany | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; NAT. REGISTRATION NO/DATE: 7002248.00.00 20210426; FIRST REGISTRATION: DAENEMARK 61678 20191016 |
| 2588114 | 2020C/518 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; AUTHORISATION NUMBER AND DATE: BE548284 20191107 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


