ZYKADIA Drug Patent Profile
✉ Email this page to a colleague
When do Zykadia patents expire, and what generic alternatives are available?
Zykadia is a drug marketed by Novartis and is included in two NDAs. There are seven patents protecting this drug.
This drug has two hundred and eighty-six patent family members in fifty-four countries.
The generic ingredient in ZYKADIA is ceritinib. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the ceritinib profile page.
DrugPatentWatch® Generic Entry Outlook for Zykadia
Zykadia was eligible for patent challenges on April 29, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 18, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ZYKADIA
| International Patents: | 286 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 98 |
| Clinical Trials: | 11 |
| Patent Applications: | 761 |
| Drug Prices: | Drug price information for ZYKADIA |
| What excipients (inactive ingredients) are in ZYKADIA? | ZYKADIA excipients list |
| DailyMed Link: | ZYKADIA at DailyMed |
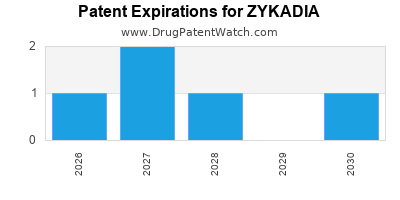

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZYKADIA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZYKADIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| CIC-EC 1401/EUCLID | Phase 3 |
| Institut National de la Santé Et de la Recherche Médicale, France | Phase 3 |
| Plateforme labellisée Inca - Institut Bergonié, Bordeaux | Phase 3 |
Pharmacology for ZYKADIA
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 2C9 Inhibitors Cytochrome P450 3A Inhibitors Tyrosine Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ZYKADIA
US Patents and Regulatory Information for ZYKADIA
ZYKADIA is protected by seven US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZYKADIA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ZYKADIA
2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
2,4-di (phenylamino) pyrimidines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compounds and compositions as protein kinase inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compounds and compositions as protein kinase inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A CANCER MEDIATED BY AN ANAPLASTIC LYMPHOMA KINASE (ALK)
Compounds and compositions as protein kinase inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods of using ALK inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A CANCER MEDIATED BY AN ANAPLASTIC LYMPHOMA KINASE (ALK)
Crystalline forms of 5-chloro-N2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-N4-[2-(propan- e-2-sulfonyl)-phenyl]-pyrimidine-2, 4-diamine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting ZYKADIA
TREATMENT OF PATIENTS WITH METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC) WHOSE TUMORS ARE ANAPLASTIC LYMPHOMA KINASE (ALK)-POSITIVE AS DETECTED BY AN FDA-APPROVED TEST
Exclusivity Expiration: ⤷ Sign Up
FDA HAS NOT RECOGNIZED ORPHAN-DRUG EXCLUSIVITY (ODE) FOR THIS DRUG, BUT IT CONTAINS THE SAME ACTIVE MOIETY OR MOIETIES AS ANOTHER DRUG(S) THAT WAS ELIGIBLE FOR ODE, AND ALSO SHARES ODE-PROTECTED USE(S) OR INDICATION(S) WITH THAT DRUG(S).AN APPLICATION SEEKING APPROVAL FOR THE SAME ACTIVE MOIETY OR MOIETIES, INCLUDING AN ANDA THAT CITES THIS NDA AS ITS BASIS OF SUBMISSION, MAY NOT BE APPROVED FOR SUCH ODE-PROTECTED USE(S) AND INDICATION(S)
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ZYKADIA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for ZYKADIA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Zykadia | ceritinib | EMEA/H/C/003819 Zykadia is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK) positive advanced non small cell lung cancer (NSCLC) previously treated with crizotinib. |
Authorised | no | no | no | 2015-05-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ZYKADIA
When does loss-of-exclusivity occur for ZYKADIA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4309
Estimated Expiration: ⤷ Sign Up
Patent: 2395
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 11343775
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2013015000
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 21102
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 13001723
Estimated Expiration: ⤷ Sign Up
China
Patent: 3282359
Estimated Expiration: ⤷ Sign Up
Patent: 4262324
Estimated Expiration: ⤷ Sign Up
Patent: 6008462
Estimated Expiration: ⤷ Sign Up
Patent: 6831716
Estimated Expiration: ⤷ Sign Up
Patent: 7056751
Estimated Expiration: ⤷ Sign Up
Patent: 2125884
Estimated Expiration: ⤷ Sign Up
Patent: 4989139
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 01792
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0171477
Estimated Expiration: ⤷ Sign Up
Patent: 0181737
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 19474
Estimated Expiration: ⤷ Sign Up
Patent: 21017
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 51918
Estimated Expiration: ⤷ Sign Up
Patent: 21171
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 13012770
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 51918
Estimated Expiration: ⤷ Sign Up
Patent: 21171
Estimated Expiration: ⤷ Sign Up
Patent: 53708
Estimated Expiration: ⤷ Sign Up
Guatemala
Patent: 1300153
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 41845
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 6474
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 16752
Estimated Expiration: ⤷ Sign Up
Patent: 13545812
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 51918
Estimated Expiration: ⤷ Sign Up
Patent: 21171
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 4810
Estimated Expiration: ⤷ Sign Up
Patent: 7742
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 8210
Estimated Expiration: ⤷ Sign Up
Patent: 13006952
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 771
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 0713
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 140698
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 51918
Estimated Expiration: ⤷ Sign Up
Patent: 21171
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 51918
Estimated Expiration: ⤷ Sign Up
Patent: 21171
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 99785
Estimated Expiration: ⤷ Sign Up
Patent: 46159
Estimated Expiration: ⤷ Sign Up
Patent: 13132947
Estimated Expiration: ⤷ Sign Up
Patent: 16136823
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 771
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 0856
Estimated Expiration: ⤷ Sign Up
Patent: 201510082X
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 51918
Estimated Expiration: ⤷ Sign Up
Patent: 21171
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1303599
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2325775
Estimated Expiration: ⤷ Sign Up
Patent: 130130022
Estimated Expiration: ⤷ Sign Up
Patent: 180032680
Patent: 5-클로로-N2--페닐]-피리미딘-2,4-디아민의 결정질 형태 (5--2-2--5--4--4---4[2--2--]--24- CRYSTALLINE FORMS OF 5-CHLORO-N2-2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL-N4[2-PROPANE-2-SULFONYL-PHENYL]-PYRIMIDINE-24-DIAMINE)
Estimated Expiration: ⤷ Sign Up
Patent: 190022903
Patent: 5-클로로-N2--페닐]-피리미딘-2,4-디아민의 결정질 형태 (5--2-2--5--4--4---4[2--2--]--24- CRYSTALLINE FORMS OF 5-CHLORO-N2-2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL-N4[2-PROPANE-2-SULFONYL-PHENYL]-PYRIMIDINE-24-DIAMINE)
Estimated Expiration: ⤷ Sign Up
Patent: 200039021
Patent: 5-클로로-N2--페닐]-피리미딘-2,4-디아민의 결정질 형태 (5--2-2--5--4--4---4[2--2--]--24- CRYSTALLINE FORMS OF 5-CHLORO-N2-2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL-N4[2-PROPANE-2-SULFONYL-PHENYL]-PYRIMIDINE-24-DIAMINE)
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 43016
Estimated Expiration: ⤷ Sign Up
Patent: 96526
Estimated Expiration: ⤷ Sign Up
Patent: 05973
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 76343
Estimated Expiration: ⤷ Sign Up
Patent: 76344
Estimated Expiration: ⤷ Sign Up
Patent: 1307299
Estimated Expiration: ⤷ Sign Up
Patent: 1629021
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 13000216
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZYKADIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2005516046 | ⤷ Sign Up | |
| Colombia | 5680434 | 2,4-PIRIMIDINADIAMINAS UTILES EN EL TRATAMIENTO DE ENFERMEDADES NEOPLASTICAS, DESORDENES DEL SISTEMA INMUNE E INFLAMATORIOS | ⤷ Sign Up |
| Russian Federation | 2599785 | КРИСТАЛЛИЧЕСКИЕ ФОРМЫ 5-ХЛОР-N2-(2-ИЗОПРОПОКСИ-5-МЕТИЛ-4-ПИПЕРИДИН-4-ИЛ-ФЕНИЛ)-N4-[2-(ПРОПАН-2-СУЛЬФОНИЛ)-ФЕНИЛ]-ПИРИМИДИН-2,4-ДИАМИНА (CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4-[2-(PROPANE-2-SULPHONYL)-PHENYL]-PYRIMIDINE-2,4-DIAMINE) | ⤷ Sign Up |
| Israel | 166241 | 2,4-PYRIMIDINEDIAMINE COMPOUNDS FOR USE IN THE TREATMENT OF AUTOIMMUNE DISEASES | ⤷ Sign Up |
| South Korea | 20180032680 | 5-클로로-N2--페닐]-피리미딘-2,4-디아민의 결정질 형태 (5--2-2--5--4--4---4[2--2--]--24- CRYSTALLINE FORMS OF 5-CHLORO-N2-2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL-N4[2-PROPANE-2-SULFONYL-PHENYL]-PYRIMIDINE-24-DIAMINE) | ⤷ Sign Up |
| Portugal | 2281563 | ⤷ Sign Up | |
| Brazil | 112013019643 | inibidores de alk, uso e composição farmacêutica compreendendo os mesmos | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZYKADIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2091918 | C 2015 037 | Romania | ⤷ Sign Up | PRODUCT NAME: CERITINIBSAU O SARE ACCEPTABILA FARMACEUTIC A ACESTUIA5-CLOR-N2-{(5-METIL-4-(PIPERIDIN-4-IL)-2-[(PROPAN-2-IL)OXI]FENIL-N4-[2-(PROPAN-2-SULFONIL)FENIL}PIRIMIDIN-2,4-DIAMINA; NATIONAL AUTHORISATION NUMBER: EU/1/15/999; DATE OF NATIONAL AUTHORISATION: 20150506; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/999; DATE OF FIRST AUTHORISATION IN EEA: 20150506 |
| 2091918 | 217 5020-2015 | Slovakia | ⤷ Sign Up | PRODUCT NAME: CERITINIB; REGISTRATION NO/DATE: EU/1/15/999 20150508 |
| 2091918 | 92785 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: CERITINIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI. FIRST REGISTRATION: 20150508 |
| 2091918 | CA 2015 00047 | Denmark | ⤷ Sign Up | PRODUCT NAME: CERITINIB ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/15/999 (C(2015)3218) 20150506 |
| 2091918 | 2015/047 | Ireland | ⤷ Sign Up | PRODUCT NAME: CERITINIB OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; AUTHORISATION: IRELAND EU/1/15/999 (C(2015) 3218), 20150506 |
| 2091918 | 122015000076 | Germany | ⤷ Sign Up | PRODUCT NAME: CERITINIB ODER PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/15/999 20150506 |
| 1272477 | CA 2015 00050 | Denmark | ⤷ Sign Up | PRODUCT NAME: CERITINIB ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/15/999 20150508 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


