ZYDELIG Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zydelig, and what generic alternatives are available?
Zydelig is a drug marketed by Gilead Sciences Inc and is included in one NDA. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eleven patent family members in forty countries.
The generic ingredient in ZYDELIG is idelalisib. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the idelalisib profile page.
DrugPatentWatch® Generic Entry Outlook for Zydelig
Zydelig was eligible for patent challenges on July 23, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 2, 2033. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ZYDELIG?
- What are the global sales for ZYDELIG?
- What is Average Wholesale Price for ZYDELIG?
Summary for ZYDELIG
| International Patents: | 111 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 84 |
| Clinical Trials: | 13 |
| Patent Applications: | 4,862 |
| Drug Prices: | Drug price information for ZYDELIG |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ZYDELIG |
| What excipients (inactive ingredients) are in ZYDELIG? | ZYDELIG excipients list |
| DailyMed Link: | ZYDELIG at DailyMed |
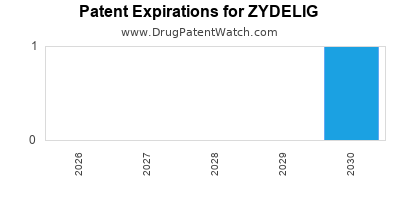

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZYDELIG
Generic Entry Date for ZYDELIG*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZYDELIG
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Loxo Oncology, Inc. | Phase 3 |
| Prospect Creek Foundation | Phase 1 |
| Oregon Health and Science University | Phase 1 |
Pharmacology for ZYDELIG
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 3A Inhibitors Kinase Inhibitors |
Paragraph IV (Patent) Challenges for ZYDELIG
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ZYDELIG | Tablets | idelalisib | 100 mg and 150 mg | 205858 | 1 | 2022-03-23 |
US Patents and Regulatory Information for ZYDELIG
ZYDELIG is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZYDELIG is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-001 | Jul 23, 2014 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-002 | Jul 23, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-001 | Jul 23, 2014 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-002 | Jul 23, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ZYDELIG
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-001 | Jul 23, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-002 | Jul 23, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-001 | Jul 23, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-002 | Jul 23, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for ZYDELIG
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Ireland UC | Zydelig | idelalisib | EMEA/H/C/003843Zydelig is indicated in combination with an anti‑CD20 monoclonal antibody (rituximab or ofatumumab) for the treatment of adult patients with chronic lymphocytic leukaemia (CLL):who have received at least one prior therapy, oras first line treatment in the presence of 17p deletion or TP53 mutation in patients who are not eligible for any other therapies.Zydelig is indicated as monotherapy for the treatment of adult patients with follicular lymphoma (FL) that is refractory to two prior lines of treatment. | Authorised | no | no | no | 2014-09-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ZYDELIG
When does loss-of-exclusivity occur for ZYDELIG?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0253
Patent: FORMAS POLIMORFICAS DE (S)-2-(1-(9H-PURIN-6-ILAMINO)PROPIL)-5-FLUOR-3-FENILQUINAZOLIN-4(3H)-ONA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 13203620
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014021935
Patent: formas polimórficas de (s)-2(l-(9h-purin-6-ilamino)propil)-5-fluoro-3-fenilquinazolina-4(3h)ona
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 64305
Patent: FORMES POLYMORPHES DE L'ACIDE -2-(1-(9H-PURINE-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE (POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14002358
Patent: Formas polimorficas de (s)-2-(1-(9h-purin-6-ilamino)propil)-5-fluoro-3-fenilquinazolin-4(3h)-ona; metodos de preparacion; composiciones farmaceuticas que las comprenden y uso en el tratamiento del cancer.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4334560
Patent: Polymorphic forms of (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one
Estimated Expiration: ⤷ Get Started Free
Patent: 6146506
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 71131
Patent: Formas polimórficas de (s)-2-(1-(9h-purin-6-ilamino)propil)-5-fluor-3-fenilquinazolin-4(3h)-ona
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 140460
Patent: FORMAS POLIMÓRFICAS DE (S)-2-(1-(9H-PURIN-6-ILAMINO)PROPIL)-5-FLUORO-3-FENILQUINAZOLIN-4(3H)-ONA
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 14020478
Patent: FORMAS POLIMÓRFICAS DE (S)-2-(1-(9H-PURIN-6-ILAMINO)PROPIL)-5-FLUORO-3-FENILQUINAZOLIN-4(3H)-ONA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 5407
Patent: ПОЛИМОРФНАЯ ФОРМА I (S)-2-(1-(9H-ПУРИН-6-ИЛАМИНО)ПРОПИЛ)-5-ФТОР-3-ФЕНИЛХИНАЗОЛИН-4(3H)-ОНА (POLYMORPHIC FORM I OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1491473
Patent: ПОЛИМОРФНЫЕ ФОРМЫ (S)-2-(1-(9H-ПУРИН-6-ИЛАМИНО)ПРОПИЛ)-5-ФТОР-3-ФЕНИЛХИНАЗОЛИН-4(3H)-ОНА
Estimated Expiration: ⤷ Get Started Free
Patent: 1690461
Patent: ПОЛИМОРФНЫЕ ФОРМЫ (S)-2-(1-(9H-ПУРИН-6-ИЛАМИНО)ПРОПИЛ)-5-ФТОР-3-ФЕНИЛХИНАЗОЛИН-4(3H)-ОНА
Estimated Expiration: ⤷ Get Started Free
Patent: 1691327
Patent: ПОЛИМОРФНЫЕ ФОРМЫ (S)-2-(1-(9H-ПУРИН-6-ИЛАМИНО)ПРОПИЛ)-5-ФТОР-3-ФЕНИЛХИНАЗОЛИН-4(3H)-ОНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 34241
Patent: FORMES POLYMORPHES DE LA (S)-2-(1-(9H-PURINE-6-YLAMINO)PROPYL)-5-FLUORO-3-PHÉNYLQUINAZOLIN-4(3H)-ONE (POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 06345
Estimated Expiration: ⤷ Get Started Free
India
Patent: 05DEN2014
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 15509537
Patent: (S)−2−(1−(9H−プリン−6−イルアミノ)プロピル)−5−フルオロ−3−フェニルキナゾリン−4(3H)−オンの多形性形態
Estimated Expiration: ⤷ Get Started Free
Patent: 16104823
Patent: (S)−2−(1−(9H−プリン−6−イルアミノ)プロピル)−5−フルオロ−3−フェニルキナゾリン−4(3H)−オンの多形性形態 (POLYMORPHIC FORM OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYL-QUINAZOLINE-4(3H)-ONE)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 14010656
Patent: FORMAS POLIMORFICAS DE (S)-2-(1-(9H-PURIN-6-ILAMINO)PROPIL)-5-FLUO RO-3-FENILQUINAZOLIN-4(3H)-ONA. (POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)- 5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE.)
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 140100
Patent: Forme polimorfe ale (S)-2-(1-(9H-purin-6-ilamino)propil)-5-fluoro-3-fenilchinazolin-4(3H)-onei;Forme polimorfe ale (S)-2-(1-(9H-purin-6-ilamino)propil)-5-fluoro-3-fenilchinazolin-4(3H)-onei (Polymorphic forms of (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 379
Patent: Formes polymorphes de l'acide -2-(1-(9h-purine-6-ylamino)propyl)-5-fluoro-3-phénylquinazolin-4(3h)-one
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9684
Patent: Polymorphic forms of (s)-2-(1-(9h-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3h)-one
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 141792
Patent: FORMAS POLIMORFICAS DE (S)-2-(1-(9H-PURIN-6-ILAMINO)PROPIL)-5-FLUOR-3-FENILQUINAZOLIN-4(3H)-ONA
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014501920
Patent: POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 34241
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 34241
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201405446P
Patent: POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 34241
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1405870
Patent: POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 140133590
Patent: POLYMORPHIC FORMS OF (S)-2-(1-(9H-PURIN-6-YLAMINO)PROPYL)-5-FLUORO-3-PHENYLQUINAZOLIN-4(3H)-ONE
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 48273
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 1350486
Patent: Polymorphic forms of (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 656
Patent: POLIMORFOS DE (S)?2?(1?(9H?PURIN?6?ILAMINO)PROPIL)?5?FLUOR?3?FENILQUINAZOLIN?4(3H)?ONA, COMPOSICIÓN Y MÉTODO DE PREPARACIÓN
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZYDELIG around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2015155436 | 血液学的な悪性疾患のための療法 (THERAPY FOR HEMATOLOGIC MALIGNANCY) | ⤷ Get Started Free |
| Australia | 2017200837 | Therapies for hematologic malignancies | ⤷ Get Started Free |
| Norway | 329136 | ⤷ Get Started Free | |
| China | 102271683 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZYDELIG
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1761540 | PA2017004 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: IDELALISIBAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; REGISTRATION NO/DATE: EU/1/14/938 20140918 |
| 1761540 | 656 | Finland | ⤷ Get Started Free | |
| 1761540 | C20170009 00226 | Estonia | ⤷ Get Started Free | PRODUCT NAME: IDELALISIIB;REG NO/DATE: EU/1/14/938 19.09.2014 |
| 1761540 | CA 2017 00007 | Denmark | ⤷ Get Started Free | PRODUCT NAME: IDELALISIB; REG. NO/DATE: EU/1/14/938 20140924 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ZYDELIG (Alectinib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
