ZALEPLON Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zaleplon, and when can generic versions of Zaleplon launch?
Zaleplon is a drug marketed by Aurobindo Pharma, Chartwell Molecular, Hikma, Hikma Pharms, Mylan, Orbion Pharms, Teva Pharms, Unichem, and Upsher Smith Labs. and is included in ten NDAs.
The generic ingredient in ZALEPLON is zaleplon. There are twelve drug master file entries for this compound. Ten suppliers are listed for this compound. Additional details are available on the zaleplon profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Zaleplon
A generic version of ZALEPLON was approved as zaleplon by AUROBINDO PHARMA on June 6th, 2008.
Summary for ZALEPLON
| US Patents: | 0 |
| Applicants: | 9 |
| NDAs: | 10 |
| Finished Product Suppliers / Packagers: | 10 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 21 |
| Patent Applications: | 4,004 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ZALEPLON |
| What excipients (inactive ingredients) are in ZALEPLON? | ZALEPLON excipients list |
| DailyMed Link: | ZALEPLON at DailyMed |
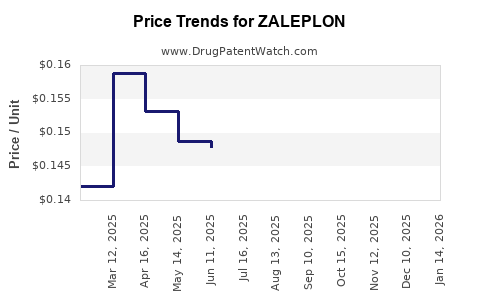
Recent Clinical Trials for ZALEPLON
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The University of Hong Kong | Phase 3 |
| Saint-Joseph University | Phase 3 |
| Taipei City Hospital | Phase 2/Phase 3 |
Pharmacology for ZALEPLON
| Drug Class | gamma-Aminobutyric Acid A Receptor Agonist |
| Mechanism of Action | GABA A Agonists |
| Physiological Effect | Central Nervous System Depression |
Anatomical Therapeutic Chemical (ATC) Classes for ZALEPLON
US Patents and Regulatory Information for ZALEPLON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurobindo Pharma | ZALEPLON | zaleplon | CAPSULE;ORAL | 078829-001 | Jun 6, 2008 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Upsher Smith Labs | ZALEPLON | zaleplon | CAPSULE;ORAL | 078706-001 | Jun 6, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Hikma Pharms | ZALEPLON | zaleplon | CAPSULE;ORAL | 078147-001 | Nov 25, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ZALEPLON
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Meda AB | Sonata | zaleplon | EMEA/H/C/000227 Sonata is indicated for the treatment of patients with insomnia who have difficulty falling asleep. It is indicated only when the disorder is severe, disabling or subjecting the individual to extreme distress. |
Withdrawn | no | no | no | 1999-03-12 | |
| Meda AB | Zerene | zaleplon | EMEA/H/C/000228 Zerene is indicated for the treatment of patients with insomnia who have difficulty falling asleep. It is indicated only when the disorder is severe, disabling or subjecting the individual to extreme distress. |
Withdrawn | no | no | no | 1999-03-12 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


