XELJANZ XR Drug Patent Profile
✉ Email this page to a colleague
When do Xeljanz Xr patents expire, and when can generic versions of Xeljanz Xr launch?
Xeljanz Xr is a drug marketed by Pfizer and is included in one NDA. There are four patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and twenty-four patent family members in fifty-five countries.
The generic ingredient in XELJANZ XR is tofacitinib citrate. There are twelve drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the tofacitinib citrate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Xeljanz Xr
A generic version of XELJANZ XR was approved as tofacitinib citrate by AJANTA PHARMA LTD on August 19th, 2025.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for XELJANZ XR?
- What are the global sales for XELJANZ XR?
- What is Average Wholesale Price for XELJANZ XR?
Summary for XELJANZ XR
| International Patents: | 124 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 30 |
| Patent Applications: | 998 |
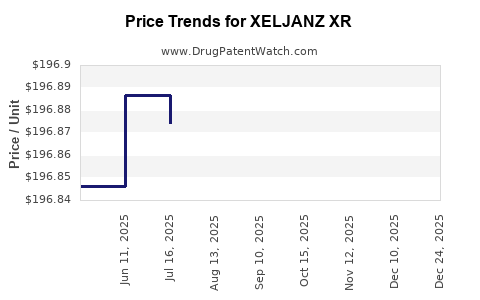
| Drug Prices: | Drug price information for XELJANZ XR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for XELJANZ XR |
| DailyMed Link: | XELJANZ XR at DailyMed |
Recent Clinical Trials for XELJANZ XR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Takeda | Phase 4 |
| Children's Hospital Los Angeles | Phase 2 |
| Fundación de Investigación Biomédica - Hospital Universitario de La Princesa | Phase 4 |
Pharmacology for XELJANZ XR
| Drug Class | Janus Kinase Inhibitor |
| Mechanism of Action | Janus Kinase Inhibitors |
Paragraph IV (Patent) Challenges for XELJANZ XR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| XELJANZ XR | Extended-release Tablets | tofacitinib citrate | 22 mg | 208246 | 1 | 2020-12-28 |
| XELJANZ XR | Extended-release Tablets | tofacitinib citrate | 11 mg | 208246 | 1 | 2016-11-07 |
US Patents and Regulatory Information for XELJANZ XR
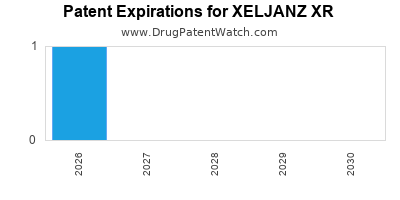
XELJANZ XR is protected by four US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for XELJANZ XR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for XELJANZ XR
When does loss-of-exclusivity occur for XELJANZ XR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5487
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 14233850
Estimated Expiration: ⤷ Get Started Free
Patent: 17203334
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015020453
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 05604
Estimated Expiration: ⤷ Get Started Free
Patent: 37328
Estimated Expiration: ⤷ Get Started Free
China
Patent: 5101952
Estimated Expiration: ⤷ Get Started Free
Patent: 1419817
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 24007
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 68155
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 68155
Estimated Expiration: ⤷ Get Started Free
Patent: 54400
Estimated Expiration: ⤷ Get Started Free
Patent: 49055
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 17297
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 53911
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1400
Estimated Expiration: ⤷ Get Started Free
Patent: 7967
Estimated Expiration: ⤷ Get Started Free
Patent: 3032
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 41823
Estimated Expiration: ⤷ Get Started Free
Patent: 14188
Estimated Expiration: ⤷ Get Started Free
Patent: 14181234
Estimated Expiration: ⤷ Get Started Free
Patent: 16199602
Estimated Expiration: ⤷ Get Started Free
Patent: 18100300
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 15013279
Estimated Expiration: ⤷ Get Started Free
Patent: 21000550
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0792
Estimated Expiration: ⤷ Get Started Free
Patent: 1227
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 68155
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 68155
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 74345
Estimated Expiration: ⤷ Get Started Free
Patent: 15139505
Estimated Expiration: ⤷ Get Started Free
Patent: 18129861
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201810985X
Estimated Expiration: ⤷ Get Started Free
Patent: 201506103U
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 68155
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1505468
Estimated Expiration: ⤷ Get Started Free
Patent: 1905100
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2151842
Estimated Expiration: ⤷ Get Started Free
Patent: 2213616
Estimated Expiration: ⤷ Get Started Free
Patent: 150131238
Estimated Expiration: ⤷ Get Started Free
Patent: 170121332
Estimated Expiration: ⤷ Get Started Free
Patent: 200103892
Estimated Expiration: ⤷ Get Started Free
Patent: 210014763
Estimated Expiration: ⤷ Get Started Free
Patent: 220151016
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 65134
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 19516
Estimated Expiration: ⤷ Get Started Free
Patent: 1436823
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering XELJANZ XR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 2968155 | ⤷ Get Started Free | |
| Panama | 8546301 | RESOLUCION DE SAL QUIRAL | ⤷ Get Started Free |
| Croatia | P20020509 | PYRROLO[2,3-d]PYRIMIDINE COMPOUNDS | ⤷ Get Started Free |
| Japan | 2003516405 | ⤷ Get Started Free | |
| European Patent Office | 1666481 | 3-((3R,4R)-4-Méthyl-3-[méthyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidinyl)-3-oxo-propionitrile comme inhibiteur de la kinase protéinique (3-((3R,4R)-4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidinyl)-3-oxo-propionitrile as protein kinase inhibitor) | ⤷ Get Started Free |
| European Patent Office | 1392694 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for XELJANZ XR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1666481 | C201730033 | Spain | ⤷ Get Started Free | PRODUCT NAME: TOFACITINIB Y SALES FARMACEUTICAMENTE ACEPTABLES DEL MISMO, INCLUYENDO LA SAL CITRATO; NATIONAL AUTHORISATION NUMBER: EU/1/17/1178; DATE OF AUTHORISATION: 20170322; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1178; DATE OF FIRST AUTHORISATION IN EEA: 20170322 |
| 1666481 | 1790038-2 | Sweden | ⤷ Get Started Free | PRODUCT NAME: TOFACITINIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE CITRATE SALT; REG. NO/DATE: EU/1/17/1178 20170324 |
| 1666481 | 37/2017 | Austria | ⤷ Get Started Free | PRODUCT NAME: TOFACITINIB, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH VERTRAEGLICHEN SALZES, EINSCHLIESSLICH DES CITRATS; REGISTRATION NO/DATE: EU/1/17/1178 (MITTEILUNG) 20170324 |
| 1666481 | 2017C/032 | Belgium | ⤷ Get Started Free | PRODUCT NAME: TOFACITINIB; AUTHORISATION NUMBER AND DATE: EU/1/17/1178 20170324 |
| 0733067 | 10299044 | Germany | ⤷ Get Started Free | PRODUCT NAME: PEGFILGRASTIM; REGISTRATION NO/DATE: EU/1/02/227/001 20020822 |
| 0733067 | 300106 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: PEGFILGRASTIM; REGISTRATION NO/DATE: EU/1/02/228/001 20020822 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for XELJANZ XR
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
