VITRAKVI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Vitrakvi, and when can generic versions of Vitrakvi launch?
Vitrakvi is a drug marketed by Bayer Hlthcare and Bayer Healthcare and is included in two NDAs. There are twenty patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and eighty-eight patent family members in fifty countries.
The generic ingredient in VITRAKVI is larotrectinib sulfate. Two suppliers are listed for this compound. Additional details are available on the larotrectinib sulfate profile page.
DrugPatentWatch® Generic Entry Outlook for Vitrakvi
Vitrakvi was eligible for patent challenges on November 26, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 15, 2036. This may change due to patent challenges or generic licensing.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VITRAKVI?
- What are the global sales for VITRAKVI?
- What is Average Wholesale Price for VITRAKVI?
Summary for VITRAKVI
| International Patents: | 288 |
| US Patents: | 20 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 121 |
| Clinical Trials: | 5 |
| Patent Applications: | 1,472 |
| Drug Prices: | Drug price information for VITRAKVI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VITRAKVI |
| What excipients (inactive ingredients) are in VITRAKVI? | VITRAKVI excipients list |
| DailyMed Link: | VITRAKVI at DailyMed |
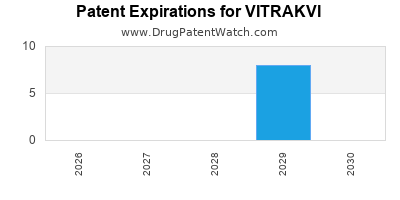

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VITRAKVI
Generic Entry Dates for VITRAKVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for VITRAKVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VITRAKVI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| M.D. Anderson Cancer Center | Phase 2 |
| Children's Oncology Group | Phase 2 |
| Genentech, Inc. | Phase 2 |
Pharmacology for VITRAKVI
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Tropomyosin Receptor Kinases Inhibitors |
Paragraph IV (Patent) Challenges for VITRAKVI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VITRAKVI | Capsules | larotrectinib sulfate | 25 mg and 100 mg | 210861 | 1 | 2025-05-06 |
US Patents and Regulatory Information for VITRAKVI
VITRAKVI is protected by twenty-one US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VITRAKVI is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,799,505.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
International Patents for VITRAKVI
When does loss-of-exclusivity occur for VITRAKVI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8090
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 15346046
Estimated Expiration: ⤷ Get Started Free
Patent: 17246547
Estimated Expiration: ⤷ Get Started Free
Patent: 17246554
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2017010141
Estimated Expiration: ⤷ Get Started Free
Patent: 2018070017
Estimated Expiration: ⤷ Get Started Free
Patent: 2018070304
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 67951
Estimated Expiration: ⤷ Get Started Free
Patent: 19661
Estimated Expiration: ⤷ Get Started Free
Patent: 19671
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 17001249
Estimated Expiration: ⤷ Get Started Free
Patent: 18002806
Estimated Expiration: ⤷ Get Started Free
Patent: 18002807
Estimated Expiration: ⤷ Get Started Free
Patent: 19002238
Estimated Expiration: ⤷ Get Started Free
Patent: 19002239
Estimated Expiration: ⤷ Get Started Free
China
Patent: 7428760
Estimated Expiration: ⤷ Get Started Free
Patent: 9310694
Estimated Expiration: ⤷ Get Started Free
Patent: 9414442
Estimated Expiration: ⤷ Get Started Free
Patent: 3354649
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 17005483
Estimated Expiration: ⤷ Get Started Free
Patent: 18010761
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 170263
Estimated Expiration: ⤷ Get Started Free
Patent: 180501
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0230271
Estimated Expiration: ⤷ Get Started Free
Patent: 0241282
Estimated Expiration: ⤷ Get Started Free
Patent: 0241295
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 180125
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 018000214
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 18083443
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 18380
Estimated Expiration: ⤷ Get Started Free
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0227339
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 44483
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 61448
Estimated Expiration: ⤷ Get Started Free
Patent: 68542
Estimated Expiration: ⤷ Get Started Free
Patent: 68971
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2270
Estimated Expiration: ⤷ Get Started Free
Patent: 2003
Estimated Expiration: ⤷ Get Started Free
Patent: 2005
Estimated Expiration: ⤷ Get Started Free
Patent: 0905
Estimated Expiration: ⤷ Get Started Free
Patent: 4018
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 14834
Estimated Expiration: ⤷ Get Started Free
Patent: 57343
Estimated Expiration: ⤷ Get Started Free
Patent: 61602
Estimated Expiration: ⤷ Get Started Free
Patent: 17535550
Estimated Expiration: ⤷ Get Started Free
Patent: 19510827
Estimated Expiration: ⤷ Get Started Free
Patent: 19511575
Estimated Expiration: ⤷ Get Started Free
Patent: 21169496
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6478
Estimated Expiration: ⤷ Get Started Free
Patent: 6415
Estimated Expiration: ⤷ Get Started Free
Patent: 6416
Estimated Expiration: ⤷ Get Started Free
Patent: 17006412
Estimated Expiration: ⤷ Get Started Free
Patent: 18012163
Estimated Expiration: ⤷ Get Started Free
Patent: 18012165
Estimated Expiration: ⤷ Get Started Free
Patent: 20011079
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 601
Estimated Expiration: ⤷ Get Started Free
Patent: 610
Estimated Expiration: ⤷ Get Started Free
Patent: 612
Estimated Expiration: ⤷ Get Started Free
Patent: 504
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1909
Estimated Expiration: ⤷ Get Started Free
Patent: 7135
Estimated Expiration: ⤷ Get Started Free
Patent: 7140
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1800102
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 181888
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 017500900
Estimated Expiration: ⤷ Get Started Free
Patent: 018502124
Estimated Expiration: ⤷ Get Started Free
Patent: 018502134
Estimated Expiration: ⤷ Get Started Free
Patent: 021550809
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 23990
Estimated Expiration: ⤷ Get Started Free
Patent: 51636
Patent: СПОСОБЫ ЛЕЧЕНИЯ ДЕТСКИХ РАКОВЫХ ЗАБОЛЕВАНИЙ (METHODS FOR TREATMENT OF CHILDHOOD CANCER DISEASES)
Estimated Expiration: ⤷ Get Started Free
Patent: 51767
Patent: ЖИДКИЕ СОСТАВЫ (S)-N-(5-((R)-2-(2,5-ДИФТОРФЕНИЛ)ПИРРОЛИДИН-1-ИЛ)ПИРАЗОЛО[1,5-A]ПИРИМИДИН-3-ИЛ)-3-ГИДРОКСИПИРРОЛИДИН-1-КАРБОКСАМИДА (LIQUID COMPOSITIONS (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)PYRROLIDINE-1-YL)PYRAZOLO[1,5-A]PYRIMIDINE-3-YL)-3-HYDROXYPYRROLIDINE-1-ARBOXAMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 17120846
Estimated Expiration: ⤷ Get Started Free
Patent: 18137206
Patent: ЖИДКИЕ СОСТАВЫ (S)-N-(5-((R)-2-(2,5-ДИФТОРФЕНИЛ)ПИРРОЛИДИН-1-ИЛ)ПИРАЗОЛО[1,5-A]ПИРИМИДИН-3-ИЛ)-3-ГИДРОКСИПИРРОЛИДИН-1-КАРБОКСАМИДА
Estimated Expiration: ⤷ Get Started Free
Patent: 18138579
Patent: СПОСОБЫ ЛЕЧЕНИЯ ДЕТСКИХ РАКОВЫХ ЗАБОЛЕВАНИЙ
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 8400168
Patent: صيغ سائلة من (S)-N-(5-((R)-2-(2، 5-داي فلورو فينيل)-بيروليدين-1-يل)-بيرازولو[1، 5-A] بيريميدين-3-يل)-3-هيدروكسي بيروليدين-1-كربوكساميد (LIQUID FORMULATIONS OF (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE)
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 122
Estimated Expiration: ⤷ Get Started Free
Patent: 987
Estimated Expiration: ⤷ Get Started Free
Patent: 988
Patent: POSTUPAK LEČENJA PEDIJATRIJSKIH KARCINOMA (METHODS OF TREATING PEDIATRIC CANCERS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201703962X
Estimated Expiration: ⤷ Get Started Free
Patent: 201808559P
Patent: LIQUID FORMULATIONS OF (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE
Estimated Expiration: ⤷ Get Started Free
Patent: 201808676R
Patent: METHODS OF TREATING PEDIATRIC CANCERS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 39662
Estimated Expiration: ⤷ Get Started Free
Patent: 39663
Estimated Expiration: ⤷ Get Started Free
Patent: 99181
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1703501
Estimated Expiration: ⤷ Get Started Free
Patent: 1806684
Patent: METHODS OF TREATING PEDIATRIC CANCERS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2400423
Estimated Expiration: ⤷ Get Started Free
Patent: 2649887
Estimated Expiration: ⤷ Get Started Free
Patent: 170082628
Estimated Expiration: ⤷ Get Started Free
Patent: 180128484
Estimated Expiration: ⤷ Get Started Free
Patent: 180129911
Estimated Expiration: ⤷ Get Started Free
Patent: 210010652
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 41630
Estimated Expiration: ⤷ Get Started Free
Patent: 87474
Estimated Expiration: ⤷ Get Started Free
Patent: 87501
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 46426
Estimated Expiration: ⤷ Get Started Free
Patent: 46537
Estimated Expiration: ⤷ Get Started Free
Patent: 67858
Estimated Expiration: ⤷ Get Started Free
Patent: 1625636
Estimated Expiration: ⤷ Get Started Free
Patent: 1801730
Patent: Liquid formulations of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-A]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide
Estimated Expiration: ⤷ Get Started Free
Patent: 2206436
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 18000335
Patent: LIQUID FORMULATIONS OF (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE
Estimated Expiration: ⤷ Get Started Free
Patent: 18000338
Patent: METHODS OF TREATING PEDIATRIC CANCERS
Estimated Expiration: ⤷ Get Started Free
Patent: 19000332
Patent: METHODS OF TREATING PEDIATRIC CANCERS
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 3044
Estimated Expiration: ⤷ Get Started Free
Patent: 5025
Patent: СПОСОБИ ЛІКУВАННЯ ДИТЯЧИХ РАКОВИХ ЗАХВОРЮВАНЬ (METHODS OF TREATING PEDIATRIC CANCERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 5026
Patent: РІДКІ КОМПОЗИЦІЇ (S)-N-(5-((R)-2-(2,5-ДИФТОРФЕНІЛ)ПІРОЛІДИН-1-ІЛ)ПІРАЗОЛО[1,5-a]ПІРИМІДИН-3-ІЛ)-3-ГІДРОКСИПІРОЛІДИН-1-КАРБОКСАМІДУ (LIQUID FORMULATIONS OF (S)-N-(5-(R)-2-(2,5-DIFLUOROPHENYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VITRAKVI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Nicaragua | 201800102 | FORMULACIONES LÍQUIDAS DE (S)-N-(5-((R)-2-(2,5-DIFLUOROFENIL)-PIRROLIDIN-1-IL)-PIRAZOLO[1, 5-A]PIRIMIDIN-3-IL)-3-HIDROXIPIRROLIDINA-1-CARBOXAMIDA. | ⤷ Get Started Free |
| Russian Federation | 2523544 | ЗАМЕЩЕННЫЕ ПИРАЗОЛО[1,5-a]ПИРИМИДИНОВЫЕ СОЕДИНЕНИЯ КАК ИНГИБИТОРЫ ТРК КИНАЗЫ (SUBSTITUTED PYRAZOLO[1,5-a]PYRIMIDINE COMPOUNDS AS TROPOMYOSIN-RELATED KINASE INHIBITORS) | ⤷ Get Started Free |
| Russian Federation | 2011120345 | ⤷ Get Started Free | |
| Dominican Republic | P2018000214 | ⤷ Get Started Free | |
| Mexico | 2017006412 | FORMA CRISTALINA DE SULFATO ACIDO DE (S)-N-(5-((R)-2-(2,5-DIFLUORO FENIL)-PIRROLIDIN-1-IL)-PIRAZOLO[1,5-A]-PIRIMIDIN-3-IL)-3-HIDROXI PIRROLIDINA-1-CARBOXAMIDA. (CRYSTALLINE FORM OF (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)-PYRROLID IN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-C ARBOXAMIDE HYDROGEN SULFATE.) | ⤷ Get Started Free |
| Philippines | 12021550809 | CRYSTALLINE FORM OF (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE HYDROGEN SULFATE | ⤷ Get Started Free |
| Colombia | 6440551 | COMPUESTOS PIRAZOLO[1,5-A]PIRIMIDINA SUSTITUIDA COMO INHIBIDORES DE TRK CINASA | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VITRAKVI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3106463 | SPC/GB20/013 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: LAROTRECITINIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, PARTICULARLY LAROTRECITINIB SULFATE INCLUDING LAROTRECITINIB HYDROGEN SULFATE; REGISTERED: UK EU/1/19/1385(NI) 20190923; UK PLGB 00010/0741 20190923; UK PLGB 00010/0742 20190923; UK PLGB 00010/0743 20190923 |
| 3106463 | 722 | Finland | ⤷ Get Started Free | |
| 3106463 | 2090009-8 | Sweden | ⤷ Get Started Free | PRODUCT NAME: LAROTRECTINIB,OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, PARTICULARLY LAROTRECTINIB SULFATE INCLUDING LAROTRECTINIB HYDROGEN SULFATE; REG. NO/DATE: EU/1/19/1385 20190923 |
| 3106463 | 2020/009 | Ireland | ⤷ Get Started Free | PRODUCT NAME: LAROTRECTINIB AND/OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, PARTICULARLY LAROTRECTINIB SULFATE INCLUDING LAROTRECTINIB HYDROGEN SULFATE; REGISTRATION NO/DATE: EU/1/19/1385 20190923 |
| 3106463 | 122020000012 | Germany | ⤷ Get Started Free | PRODUCT NAME: LAROTRECTINIB ODER ANDERE PHARMAZEUTISCH ANNEHMBARE SALZE DAVON, EINSCHLIESSLICH LAROTRECTINIBSULFATE UMFASSEND LAROTRECTINIBHYDROGENSULFAT; REGISTRATION NO/DATE: EU/1/19/1385 20190919 |
| 3106463 | C20200005 00308 | Estonia | ⤷ Get Started Free | PRODUCT NAME: LAROTREKTINIIB;REG NO/DATE: EU/1/19/1385; 23.09.2019 |
| 3106463 | 301033 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: LAROTRECTINIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER LAROTRECTINIBSULFAAT, MET INBEGRIP VAN LAROTRECTINIBSULFAAT MONOHYDRAAT; REGISTRATION NO/DATE: EU/1/19/1385 20190923 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for VITRAKVI (Larotrectinib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
