TRINTELLIX Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Trintellix, and when can generic versions of Trintellix launch?
Trintellix is a drug marketed by Takeda Pharms Usa and is included in one NDA. There are ten patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and seventeen patent family members in forty-two countries.
The generic ingredient in TRINTELLIX is vortioxetine hydrobromide. There are sixteen drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the vortioxetine hydrobromide profile page.
DrugPatentWatch® Generic Entry Outlook for Trintellix
Trintellix was eligible for patent challenges on September 30, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 30, 2031. This may change due to patent challenges or generic licensing.
There have been twenty-four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (vortioxetine hydrobromide), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TRINTELLIX?
- What are the global sales for TRINTELLIX?
- What is Average Wholesale Price for TRINTELLIX?
Summary for TRINTELLIX
| International Patents: | 217 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 83 |
| Clinical Trials: | 11 |
| Patent Applications: | 223 |
| Drug Prices: | Drug price information for TRINTELLIX |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRINTELLIX |
| What excipients (inactive ingredients) are in TRINTELLIX? | TRINTELLIX excipients list |
| DailyMed Link: | TRINTELLIX at DailyMed |
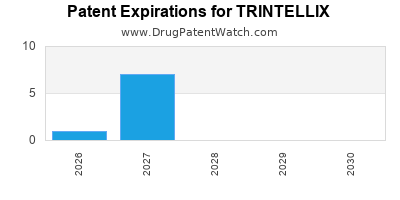
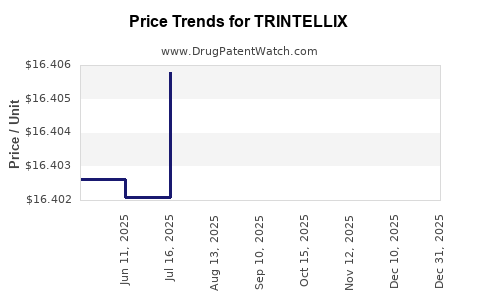
See drug prices for TRINTELLIX
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TRINTELLIX
Generic Entry Date for TRINTELLIX*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TRINTELLIX
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Seasons Biotechnology (Taizhou) Co., Ltd. | Phase 1 |
| H. Lundbeck A/S | Phase 3 |
| Todd Doyle | Phase 4 |
Paragraph IV (Patent) Challenges for TRINTELLIX
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRINTELLIX | Tablets | vortioxetine hydrobromide | 5 mg, 10 mg, 15 mg and 20 mg | 204447 | 15 | 2017-10-02 |
US Patents and Regulatory Information for TRINTELLIX
TRINTELLIX is protected by ten US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TRINTELLIX is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for TRINTELLIX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-004 | Sep 30, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-001 | Sep 30, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-003 | Sep 30, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-002 | Sep 30, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRINTELLIX
When does loss-of-exclusivity occur for TRINTELLIX?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1481
Patent: COMPUESTOS CON ACTIVIDAD COMBINADA SOBRE SERT, 5-HT3 Y 5-HT1A
Estimated Expiration: ⤷ Get Started Free
Patent: 5797
Patent: USOS Y DERIVADOS DE 1-(2- (2,4- DIMETILFENILSULFANIL) FENIL) PIPERAZINA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07260355
Patent: 1- [2- (2, 4-dimethylphenylsulfanyl) -phenyl] piperazine as a compound with combined serotonin reuptake, 5-HT3 and 5-HT1A activity for the treatment of cognitive impairment
Estimated Expiration: ⤷ Get Started Free
Patent: 08228638
Patent: 1- [2-(2,4-dimethylphenylsulfanyl)-phenyl] piperazine as a compound with combined serotonin reuptake, 5-HT3 and 5-HT1A activity for the treatment of pain or residual symptoms in depression relating to sleep and cognition
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 95745
Estimated Expiration: ⤷ Get Started Free
Patent: 40941
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0713425
Patent: composto, composição farmacêutica, método para tratar uma doença, uso de um composto, e processos para a preparação de um composto, e para a fabricação de um composto
Estimated Expiration: ⤷ Get Started Free
Patent: 0808941
Patent: MÉTODO PARA O TRATAMENTO DE DOR OU SINTOMAS RESIDUAIS EM DEPRESSÃO, USO DE UM COMPOSTO, E, COMPOSTO
Estimated Expiration: ⤷ Get Started Free
Patent: 2020011899
Patent: processos para a preparação de 1-[2-(2,4-dimetil-fenilsulfanil)-fenil]-piperazina e para a fabricação do sal de adição de ácido bromídrico correspondente
Estimated Expiration: ⤷ Get Started Free
Patent: 2020011920
Patent: composto, composição farmacêutica, método para tratar uma doença, uso de um composto, e processos para a preparação de um composto, e para a fabricação de um composto
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 55212
Patent: 1-[2-(2, 4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE COMME COMPOSE PRESENTANT UNE ACTIVITE SUR LA SEROTONINE, 5-HT3 ET 5-HT1A POUR LE TRAITEMENT DU DEFICIT COGNITIF (1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 84571
Patent: NOUVELLES UTILISATIONS THERAPEUTIQUES DE 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)PHENYL]PIPERAZINE (NOVEL THERAPEUTIC USES OF 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)PHENYL]-PIPE RAZINE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 08000795
Patent: USO DE 1-[2-(2,4-DIMETILFENILSULFANIL)FENIL)PIPERAZINA PARA EL TRATAMIENTO DEL DOLOR O DE LOS SINTOMAS RESIDUALES DE LA DEPRESION; Y DICHO COMPUESTO.
Estimated Expiration: ⤷ Get Started Free
Patent: 11001610
Patent: Proceso de preparacion de 1-[2-(2,4-dimetilfenilsulfanil)-fenil]piperazina (div. sol. 1758-07).
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1472906
Patent: 1- [2- (2, 4-dimethylphenylsulfanyl) -phenyl] piperazine as a compound with combined serotonin reuptake, 5-HT3 and 5-HT1A activity for the treatment of cognitive impairment
Estimated Expiration: ⤷ Get Started Free
Patent: 1636161
Patent: 1- [2-(2,4-dimethylphenylsulfanyl)-phenyl] piperazine as a compound with combined serotonin reuptake, 5-ht3 and 5-ht1a activity for the treatment of pain or residual symptoms in depression relating to
Estimated Expiration: ⤷ Get Started Free
Patent: 2614179
Patent: 1- [2- (2,4-dimethylphenylsulfanyl)-phenyl] piperazine as a compound with combined serotonin reuptake, 5-ht3 and 5-ht1a activity for the treatment of cognitive impairment
Estimated Expiration: ⤷ Get Started Free
Patent: 2617513
Patent: 1- [2- (2,4-dimethylphenylsulfanyl)-phenyl] piperazine as a compound with combined serotonin reuptake, 5-HT3 AND 5-HT1A activity for the treatment of cognitive impairment
Estimated Expiration: ⤷ Get Started Free
Patent: 3948597
Patent: 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine used for treatment of pain or depression residual symptoms related with sleep and cognition
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 20962
Patent: USOS TERAPEUTICOS NOVEDOSOS DE 1-[2(2,4-DIMETILFENILSULFANIL)FENIL]-PIPERAZINA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0110058
Estimated Expiration: ⤷ Get Started Free
Patent: 0120173
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 11183
Estimated Expiration: ⤷ Get Started Free
Patent: 12635
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 44043
Estimated Expiration: ⤷ Get Started Free
Patent: 42193
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 5287
Patent: 1-[2-(2,4-ДИМЕТИЛФЕНИЛСУЛЬФАНИЛ)ФЕНИЛ]ПИПЕРАЗИН В КАЧЕСТВЕ СОЕДИНЕНИЯ С СОЧЕТАНИЕМ АКТИВНОСТИ В ОТНОШЕНИИ ПОВТОРНОГО ЗАХВАТА СЕРОТОНИНА, 5-НТИ 5-НТДЛЯ ЛЕЧЕНИЯ КОГНИТИВНОГО НАРУШЕНИЯ (1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HTAND 5-HTACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 7058
Patent: НОВОЕ ТЕРАПЕВТИЧЕСКОЕ ПРИМЕНЕНИЕ 1-[2-(2,4-ДИМЕТИЛФЕНИЛСУЛЬФАНИЛ)ФЕНИЛ]ПИПЕРАЗИНА (NOVEL THERAPEUTIC USES OF 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)PHENYL]PIPERAZINE)
Estimated Expiration: ⤷ Get Started Free
Patent: 0970018
Patent: 1-[2-(2,4-ДИМЕТИЛФЕНИЛСУЛЬФАНИЛ)ФЕНИЛ]ПИПЕРАЗИН В КАЧЕСТВЕ СОЕДИНЕНИЯ С СОЧЕТАНИЕМ АКТИВНОСТИ В ОТНОШЕНИИ ПОВТОРНОГО ЗАХВАТА СЕРОТОНИНА, 5-НТ3 И 5-НТ1А ДЛЯ ЛЕЧЕНИЯ КОГНИТИВНОГО НАРУШЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 0970870
Patent: НОВОЕ ТЕРАПЕВТИЧЕСКОЕ ПРИМЕНЕНИЕ 1-[2-(2,4-ДИМЕТИЛФЕНИЛСУЛЬФАНИЛ)ФЕНИЛ]ПИПЕРАЗИНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 44043
Patent: 1-[2-(2, 4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE COMME COMPOSE PRESENTANT UNE ACTIVITE SUR LA SEROTONINE, 5-HT3 ET 5-HT1A POUR LE TRAITEMENT DU DEFICIT COGNITIF (1- Ý[- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 42193
Patent: 1-[2-(2,4-DIMÉTHYLPHÉNYLSULFANYL)PHÉNYL]PIPÉRAZINE EN TANT QUE COMPOSÉ AVEC ACTIVITÉ COMBINÉ DU RECAPTAGE DE LA SEROTONINE, 5-HT3 ET 5-HT1A POUR LE TRAITEMENT DE LA DOLEUR OU DE SYMPTÔMES RÉSIDUELLES EN DEPRESSION CONCERNANT LE SOMMEIL ET LA COGNITION (1-[2-(2,4-DIMETHYLPHENYLSULFANYL)PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF PAIN OR RESIDUAL SYMPTOMS IN DEPRESSION RELATING TO SLEEP AND COGNITION)
Estimated Expiration: ⤷ Get Started Free
Patent: 39201
Patent: COMPOSÉS AVEC ACTIVITÉ COMBINÉ SERT, 5-HT3 ET 5-HT1A (COMPOUNDS WITH COMBINED SERT, 5-HT3 AND 5-HT1A ACTIVITY)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 44043
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2008004643
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 34483
Patent: 1- [2- (2, 4-DIMETHYLPHENYLSULFANYL)-PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 71951
Patent: 作為用於治療認知損傷的、具有結合的對血清素再吸收、 活性的化合物的 -二甲基苯基硫烷基 -苯基 哌嗪 (1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT 5-HT3 5-HT1A 1-[2-(24-)-])
Estimated Expiration: ⤷ Get Started Free
Patent: 72014
Patent: 作為用於治療認知損傷的、具有結合的對血清素再吸收、 活性的化合物的 -二甲基苯基硫烷基 -苯基 哌嗪 (1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT 5-HT3 5-HT1A 1-[2-(24-)-])
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5511
Patent: 1- [2-(2, 4-דימתילפנילסולפניל)-פניל]פיפרזין כתרכובת גבישית ושימושו להכנת תרופה לטיפול בליקוי קוגניטיבי (1-[2-(2,4-dimethylphenylsulfanyl)-phenyl] piperazine which compound is crystalline and use thereof for the preparation of a medicament for treatment of cognitive impairment)
Estimated Expiration: ⤷ Get Started Free
Patent: 0956
Patent: 1-[2-(2,4-דימתילפנילסולפניל)-פניל]פיפרזין לטיפול בכאב (1-[2-(2,4-dimethylphenylsulfanyl)-phenyl]piperazine for use in the treatment of pain)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 90013
Estimated Expiration: ⤷ Get Started Free
Patent: 71790
Estimated Expiration: ⤷ Get Started Free
Patent: 02929
Estimated Expiration: ⤷ Get Started Free
Patent: 63609
Estimated Expiration: ⤷ Get Started Free
Patent: 01742
Estimated Expiration: ⤷ Get Started Free
Patent: 24082
Estimated Expiration: ⤷ Get Started Free
Patent: 79035
Estimated Expiration: ⤷ Get Started Free
Patent: 09541216
Estimated Expiration: ⤷ Get Started Free
Patent: 10090165
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 10521501
Estimated Expiration: ⤷ Get Started Free
Patent: 13056933
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL] PIPERAZINE AS COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 15157872
Patent: 認識機能障害(cognitiveimpairment)を治療するための、組み合わされたセロトニン再取り込み、5−HT3および5−HT1A活性を有する化合物としての1−[2−(2,4−ジメチルフェニルスルファニル)−フェニル]ピペラジン (1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 17008086
Patent: 認識機能障害(cognitive impairment)を治療するための、組み合わされたセロトニン再取り込み、5−HT3および5−HT1A活性を有する化合物としての1−[2−(2,4−ジメチルフェニルスルファニル)−フェニル]ピペラジン (1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS COMPOUND HAVING COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR TREATING COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 18199689
Patent: 認識機能障害(cognitive impairment)を治療するための、組み合わされたセロトニン再取り込み、5−HT3および5−HT1A活性を有する化合物としての1−[2−(2,4−ジメチルフェニルスルファニル)−フェニル]ピペラジン (1-[2[(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS COMPOUND WITH COMBINED SEROTONIN REUPTAKE 5-HT3 AND 5-HT1A ACTIVITY FOR TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 20152732
Patent: 認識機能障害(cognitive impairment)を治療するための、組み合わされたセロトニン再取り込み、5−HT3および5−HT1A活性を有する化合物としての1−[2−(2,4−ジメチルフェニルスルファニル)−フェニル]ピペラジン (1-[2[(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS COMPOUND WITH COMBINED SEROTONIN REUPTAKE 5-HT3 AND 5-HT1A ACTIVITY FOR TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 23009175
Patent: 認識機能障害(cognitive impairment)を治療するための、組み合わされたセロトニン再取り込み、5-HT3および5-HT1A活性を有する化合物としての1-[2-(2,4-ジメチルフェニルスルファニル)-フェニル]ピペラジン
Estimated Expiration: ⤷ Get Started Free
Patent: 25021476
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0448
Patent: 1- [2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF PAIN OR RESIDUAL SYMPTOMS IN DEPRESSION RELATING TO SLEEP AND COGNITION
Estimated Expiration: ⤷ Get Started Free
Patent: 0647
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 08016141
Patent: 1-[2-(2,4-DIMETILFENILSULFANIL)-FENIL] PIPERAZINA COMO UN COMPUESTO CON ACTIVIDAD COMBINADA DE RECAPTACIÓN DE SEROTONINA, 5-HT3 Y 5-HT1A.PARA EL TRATAMIENTO DE DAÑO COGNITIVO. (1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT.)
Estimated Expiration: ⤷ Get Started Free
Patent: 09009672
Patent: COMPUESTO 1-[2-(2,4-DIMETILFENILSULFANIL)-FENIL]PIPERAZINA EN COMBINACION CON SEROTONINA PARA LA ACTIVIDAD DE RECAPTACION, 5-HT3 Y 5-HT1 PARA EL TRATAIENTO DEL DOLOR O LOS SINTOMAS RESIDUALES EN LA DEPRESION RELACIONADOS CON EL SUEÑO Y LA COGNICION. (1- [2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF PAIN OR RESIDUAL SYMPTOMS IN DEPRESSION RELATING TO SLEEP AND COGNITION.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 575
Patent: 1-[2-(2, 4-DIMÉTHYLPHÉNYLSULFANYL)-PHÉNYL]PIPÉRAZINE COMME COMPOSÉ PRÉSENTANT UNE ACTIVITÉ SUR LA SÉROTONINE, 5-HT3 ET 5-HT1A POUR LE TRAITEMENT DU DÉFICIT COGNITIF
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2986
Patent: 1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 9721
Patent: 1-[2-(2,4-dimethylphenylsulfanyl)-phenyl] piperazine as a compound with combined serotonin reuptake, 5-HT3 and 5-HT1A activity for the treatment of pain or residual symptoms in depression relating to sleep and cognition
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 3929
Estimated Expiration: ⤷ Get Started Free
Patent: 090229
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 44043
Estimated Expiration: ⤷ Get Started Free
Patent: 42193
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 44043
Estimated Expiration: ⤷ Get Started Free
Patent: 42193
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 560
Patent: 1-[2-(2,4-DIMETILFENILSULFANIL)FENIL]PIPERAZIN KAO JEDINJENJE SA KOMBINOVANOM AKTIVNOŠĆU VEZANOM ZA PONOVNO PREUZIMANJE SEROTONINA I 5-HT3 I 5HT1A AKTIVNOŠĆU, ZA LEČENJE BOLA ILI REZIDUALNIH SIMPTOMA U DEPRESIJI VEZANIH ZA SAN I KOGNICIJU (1-[2-(2,4-DIMETHYLPHENYLSULFANYL)PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF PAIN OR RESIDUAL SYMPTOMS IN DEPRESSION RELATING TO SLEEP AND COGNITION)
Estimated Expiration: ⤷ Get Started Free
Patent: 205
Patent: 1-[2-(2,4-DIMETILFENILSULFANIL)-FENIL]PIPERAZIN KAO JEDINJENJE SA KOMBINOVANOM AKTIVNOŠĆU PONOVNOG UZIMANJA SEROTONINA, 5-HT3 I 5-HT1A, ZA TRETMAN KOGNITIVNIH OŠTEĆENJA (1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 44043
Estimated Expiration: ⤷ Get Started Free
Patent: 42193
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0810017
Patent: 1- [2- (2, 4-Dimethylphenylsulfanyl) -phenyl] piperazine as a compound with combined serotonin Reuptake, 5-HT3 and 5-HT1A activity for the treatment of cognitive impairment
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1445514
Estimated Expiration: ⤷ Get Started Free
Patent: 1459168
Estimated Expiration: ⤷ Get Started Free
Patent: 1627901
Estimated Expiration: ⤷ Get Started Free
Patent: 090028712
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 090125251
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF PAIN OR RESIDUAL SYMPTOMS IN DEPRESSION RELATING TO SLEEP AND COGNITION
Estimated Expiration: ⤷ Get Started Free
Patent: 130079619
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 130133078
Patent: 1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 55434
Estimated Expiration: ⤷ Get Started Free
Patent: 79200
Estimated Expiration: ⤷ Get Started Free
Patent: 32102
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 43091
Estimated Expiration: ⤷ Get Started Free
Patent: 0817340
Patent: Compounds with combined SERT, 5-HT3 and 5-HT1A activity
Estimated Expiration: ⤷ Get Started Free
Patent: 0848411
Patent: Novel therapeutic uses of 1-[2-(2, 4-dimethylphenylsulfanyl)phenyl]-piperazine
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 08460
Patent: 1- [2- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL] PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 250
Patent: КРИСТАЛЛИЧЕСКАЯ ФОРМА ПРОИЗВОДНЫХ 1-[2-(2,4-ДИМЕТИЛФЕНИЛСУЛЬФАНИЛ)-ФЕНИЛ]ПИПЕРАЗИНА KAK СОЕДИНЕНИЕ C КОМБИНИРОВАННОЙ АКТИВНОСТЬЮ B ОТНОШЕНИИ ОБРАТНОГО ЗАХВАТА СЕРОТОНИНА, 5-HT3 И 5-HT1A ДЛЯ ЛЕЧЕНИЯ КОГНИТИВНЫХ НАРУШЕНИЙ;КРИСТАЛІЧНА ФОРМА ПОХІДНИХ 1-[2-(2,4-ДИМЕТИЛФЕНІЛСУЛЬФАНІЛ)-ФЕНІЛ]ПІПЕРАЗИНУ ЯК СПОЛУКА З КОМБІНОВАНОЮ АКТИВНІСТЮ СТОСОВНО ЗВОРОТНОГО ЗАХОПЛЕННЯ СЕРОТОНІНУ, 5-HT3 ТА 5-HT1A ДЛЯ ЛІКУВАННЯ КОГНІТИВНИХ ПОРУШЕНЬ (CRYSTALLINE FORM OF 1- [2- (2,4-DIMETHYLPHENYLSULFANYL)-PHENYL] PIPERAZINE DERIVATIVES AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 392
Patent: 1-[2-(2,4-ДИМЕТИЛФЕНИЛСУЛЬФАНИЛ)ФЕНИЛ]ПИПЕРАЗИН КАК СОЕДИНЕНИЕ С КОМБИНИРОВАННОЙ АКТИВНОСТЬЮ В ОТНОШЕНИИ ОБРАТНОГО ЗАХВАТА СЕРОТОНИНА, 5-НТ3 И 5-НТ1A ДЛЯ ЛЕЧЕНИЯ БОЛИ;1-[2-(2,4-ДИМЕТИЛФЕНІЛСУЛЬФАНІЛ)ФЕНІЛ]ПІПЕРАЗИН ЯК СПОЛУКА З КОМБІНОВАНОЮ АКТИВНІСТЮ СТОСОВНО ЗВОРОТНОГО ЗАХОПЛЕННЯ СЕРОТОНІНУ, 5-HT3 ТА 5-HT1A ДЛЯ ЛІКУВАННЯ БОЛЮ (1-[2-(2,4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF PAIN)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRINTELLIX around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 103948597 | 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine used for treatment of pain or depression residual symptoms related with sleep and cognition | ⤷ Get Started Free |
| Poland | 2142193 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 015287 | ⤷ Get Started Free | |
| Chile | 2008000795 | USO DE 1-[2-(2,4-DIMETILFENILSULFANIL)FENIL)PIPERAZINA PARA EL TRATAMIENTO DEL DOLOR O DE LOS SINTOMAS RESIDUALES DE LA DEPRESION; Y DICHO COMPUESTO. | ⤷ Get Started Free |
| South Africa | 200810017 | 1- [2- (2, 4-Dimethylphenylsulfanyl) -phenyl] piperazine as a compound with combined serotonin Reuptake, 5-HT3 and 5-HT1A activity for the treatment of cognitive impairment | ⤷ Get Started Free |
| Norway | 2014011 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRINTELLIX
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1436271 | 14C0033 | France | ⤷ Get Started Free | PRODUCT NAME: VORTIOXETINE OU L'UN DE SES SELS D'ADDITION D'ACIDE PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/13/891 20131218 |
| 1436271 | 302 50003-2014 | Slovakia | ⤷ Get Started Free | OWNER(S): H. LUNDBECK A/S, VALBY, DK |
| 1436271 | C300652 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: VORTIOXETINE OF EEN; REGISTRATION NO/DATE: EU/1/13/891/001-036 20131218 |
| 1436271 | 2014C/036 | Belgium | ⤷ Get Started Free | PRODUCT NAME: VORTIOXETINE; AUTHORISATION NUMBER AND DATE: EU/1/13/891 20131220 |
| 1436271 | PA2014013 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: VORTIOXETINUM; REGISTRATION NO/DATE: EU/1/13/891 20131218 |
| 1436271 | 122014000049 | Germany | ⤷ Get Started Free | PRODUCT NAME: VORTIOXETIN ODER EIN PHARMAZEUTISCH AKZEPTABLES SAEUREADDITIONSSALZ DAVON; REGISTRATION NO/DATE: EU/1/13/891 20131218 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
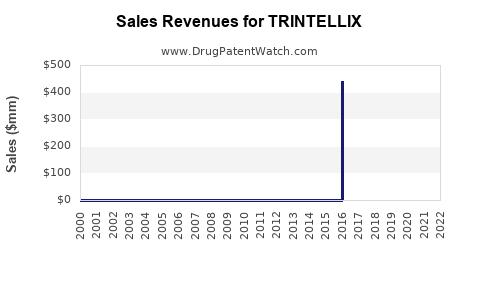
Market Dynamics and Financial Trajectory for Trintellix (Vortioxetine)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
