TPOXX Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tpoxx, and when can generic versions of Tpoxx launch?
Tpoxx is a drug marketed by Siga Technologies and is included in two NDAs. There are eight patents protecting this drug.
This drug has ninety-six patent family members in twenty-four countries.
The generic ingredient in TPOXX is tecovirimat. One supplier is listed for this compound. Additional details are available on the tecovirimat profile page.
DrugPatentWatch® Generic Entry Outlook for Tpoxx
Tpoxx was eligible for patent challenges on July 13, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 23, 2031. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TPOXX?
- What are the global sales for TPOXX?
- What is Average Wholesale Price for TPOXX?
Summary for TPOXX
| International Patents: | 96 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Patent Applications: | 111 |
| What excipients (inactive ingredients) are in TPOXX? | TPOXX excipients list |
| DailyMed Link: | TPOXX at DailyMed |
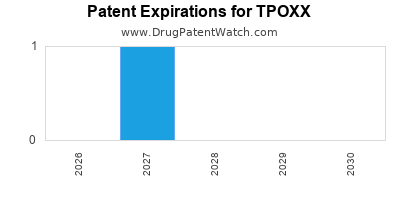
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TPOXX
Generic Entry Dates for TPOXX*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for TPOXX*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for TPOXX
US Patents and Regulatory Information for TPOXX
TPOXX is protected by nine US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TPOXX is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,339,466.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siga Technologies | TPOXX | tecovirimat | CAPSULE;ORAL | 208627-001 | Jul 13, 2018 | RX | Yes | Yes | 7,737,168 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Siga Technologies | TPOXX | tecovirimat | SOLUTION;INTRAVENOUS | 214518-001 | May 18, 2022 | RX | Yes | Yes | 7,737,168 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Siga Technologies | TPOXX | tecovirimat | CAPSULE;ORAL | 208627-001 | Jul 13, 2018 | RX | Yes | Yes | 12,433,868 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TPOXX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Siga Technologies | TPOXX | tecovirimat | SOLUTION;INTRAVENOUS | 214518-001 | May 18, 2022 | 8,530,509 | ⤷ Get Started Free |
| Siga Technologies | TPOXX | tecovirimat | CAPSULE;ORAL | 208627-001 | Jul 13, 2018 | 8,124,643 | ⤷ Get Started Free |
| Siga Technologies | TPOXX | tecovirimat | SOLUTION;INTRAVENOUS | 214518-001 | May 18, 2022 | 8,802,714 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TPOXX
When does loss-of-exclusivity occur for TPOXX?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 21
Patent: Polymorphic forms ST-246 and methods of preparation
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 2566
Patent: FORMULACIONES LIQUIDAS DE ST-246 Y METODOS RELACIONADOS, PROCESO
Estimated Expiration: ⤷ Get Started Free
Patent: 3435
Patent: FORMAS POLIMORFICAS DE 4-TRIFLUOROMETIL-N-(3,3A,4,4A,5,5A,6,6A-OCTAHIDRO-1,3-DIOXO-4,6-ETENCICLOPROP[F]ISOINDOL-2(1H)-IL)-BENZAMIDA
Estimated Expiration: ⤷ Get Started Free
Patent: 2178
Patent: COMPOSICIÓN FARMACÉUTICA LÍQUIDA Y PROCESO PARA PREPARARLA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 11232551
Patent: Polymorphic forms ST-246 and methods of preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 11285871
Patent: ST-246 liquid formulations and methods
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012023743
Patent: formas polimórficas st-246 e métodos de preparação
Estimated Expiration: ⤷ Get Started Free
Patent: 2013002646
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 93533
Patent: FORMES POLYMORPHES ST-246 ET PROCEDES DE PREPARATION (POLYMORPHIC FORMS OF ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 07528
Patent: PREPARATIONS LIQUIDES DE ST-246 ET PROCEDES (ST-246 LIQUID FORMULATIONS AND METHODS)
Estimated Expiration: ⤷ Get Started Free
Patent: 30671
Patent: FORMES POLYMORPHES ST-246 ET PROCEDES DE PREPARATION (POLYMORPHIC FORMS OF ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12002621
Patent: Polimorfo forma i, ii, iii, iv y vi de 4-trifluorometil-n-(3,3a,4,4a,5,5a,6,6a-octahidro-1,3-dioxo-4,6-etenocicloprop[f]isoindol-2(1h)-il)-benzamida (st-246); metodo para producirlas; composicion farmaceutica; forma de dosificacion unitaria; y metodo de tratamiento de infecciones por ortopoxvirus y de eczema vacunal.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3068232
Patent: Polymorphic forms st-246 and methods of preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 3281898
Patent: St-246 liquid formulations and methods
Estimated Expiration: ⤷ Get Started Free
Patent: 5111130
Patent: Polymorphic forms of ST-246 and methods of preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 5111131
Patent: Polymorphic forms of ST-246 and methods of preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 5175311
Patent: Polymorphic Forms of ST-246 and Methods of Preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 6074370
Patent: ST‑246液体配制物以及方法 (ST-246 LIQUID FORMULATIONS AND METHODS)
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 49871
Estimated Expiration: ⤷ Get Started Free
Patent: 00715
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 49871
Patent: FORME POLYMORPHE ST-246 ET PROCÉDÉS DE PRÉPARATION (POLYMORPHIC FORM ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 00715
Patent: PRÉPARATIONS LIQUIDES DE ST-246 ET PROCÉDÉS (ST-246 LIQUID FORMULATIONS AND METHODS)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1023
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1991
Patent: תצורות פולימורפיות של 246-st ושיטות להכנתן (Polymorphic forms st-246 and methods of preparation)
Estimated Expiration: ⤷ Get Started Free
Patent: 4430
Patent: Liquid pharmaceutical formulation comprising st-246 and a beta-cyclodextrin, a process of making the liquid formulation, a unit dosage liquid formulation and a process for preparing a water-soluble solid st-246 pharmaceutical formulation
Estimated Expiration: ⤷ Get Started Free
Patent: 8239
Patent: ושיטות st-תוצרות פולימורפיות של 246 להכנתן (Polymorphic forms st-246 and methods of preparation)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 98196
Estimated Expiration: ⤷ Get Started Free
Patent: 18041
Estimated Expiration: ⤷ Get Started Free
Patent: 13522371
Estimated Expiration: ⤷ Get Started Free
Patent: 13532729
Estimated Expiration: ⤷ Get Started Free
Patent: 16040323
Patent: ST−246の多形型および調製方法 (POLYMORPHIC FORMS OF ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 18012735
Patent: ST−246の多形型および調製方法 (POLYMORPHIC FORMS OF ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 7795
Patent: FORMULACIONES LIQUIDAS DE ST-246 Y METODOS. (ST-246 LIQUID FORMULATIONS AND METHODS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 1428
Patent: FORMAS POLIMORFICAS ST-246 Y METODOS DE PREPARACION. (POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION.)
Estimated Expiration: ⤷ Get Started Free
Patent: 8106
Patent: FORMULACIONES LIQUIDAS DE ST-246 Y METODOS. (ST-246 LIQUID FORMULATIONS AND METHODS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 12010859
Patent: FORMAS POLIMORFICAS ST-246 Y METODOS DE PREPARACION. (POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13001389
Patent: FORMULACIONES LIQUIDAS DE ST-246 Y METODOS. (ST-246 LIQUID FORMULATIONS AND METHODS.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2578
Patent: Polymorphic forms st-246 and methods of preparation
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 130212
Patent: FORMAS POLIMORFICAS DE ST-246 Y METODOS DE PREPARACION
Estimated Expiration: ⤷ Get Started Free
Patent: 170944
Patent: FORMAS POLIMORFICAS DE ST-246 Y METODOS DE PREPARACION
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 78606
Patent: ПОЛИМОРФНЫЕ ФОРМЫ СОЕДИНЕНИЯ ST-246 И СПОСОБЫ ПОЛУЧЕНИЯ (POLYMORPHIC FORMS OF ST-246 COMPOUND AND METHODS FOR PRODUCING)
Estimated Expiration: ⤷ Get Started Free
Patent: 12144818
Patent: ПОЛИМОРФНЫЕ ФОРМЫ СОЕДИНЕНИЯ ST-246 И СПОСОБЫ ПОЛУЧЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 15146899
Patent: ПОЛИМОРФНЫЕ ФОРМЫ СОЕДИНЕНИЯ ST-246 И СПОСОБЫ ПОЛУЧЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 4201
Patent: POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 7243
Patent: ST-246 LIQUID FORMULATIONS AND METHODS
Estimated Expiration: ⤷ Get Started Free
Patent: 201501936P
Patent: POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 201506031U
Patent: ST-246 LIQUID FORMULATIONS AND METHODS
Estimated Expiration: ⤷ Get Started Free
Patent: 202105262Q
Patent: ST-246 LIQUID FORMULATIONS AND METHODS
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1207141
Patent: POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 1300930
Patent: ST-246 LIQUID FORMULATIONS AND METHODS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1868117
Estimated Expiration: ⤷ Get Started Free
Patent: 130018271
Patent: POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 130135836
Patent: ST-246 LIQUID FORMULATIONS AND METHODS
Estimated Expiration: ⤷ Get Started Free
Patent: 150011016
Patent: POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 150092354
Patent: POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 160028489
Patent: 다형체 형태 ST―246 및 제조방법 (POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 160062208
Patent: 다형체 형태 ST―246 및 제조방법 (POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 170102070
Patent: 다형체 형태 ST―246 및 제조방법 (POLYMORPHIC FORMS ST-246 AND METHODS OF PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TPOXX around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Africa | 201300930 | ST-246 LIQUID FORMULATIONS AND METHODS | ⤷ Get Started Free |
| Canada | 2793533 | FORMES POLYMORPHES ST-246 ET PROCEDES DE PREPARATION (POLYMORPHIC FORMS OF ST-246 AND METHODS OF PREPARATION) | ⤷ Get Started Free |
| European Patent Office | 2192901 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TPOXX
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1638938 | 122022000032 | Germany | ⤷ Get Started Free | PRODUCT NAME: TECOVIRIMAT ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/21/1600 20220106 |
| 1638938 | 301177 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: TECOVIRIMAT OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/21/1600/001 20220107 |
| 1638938 | 2290024-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: TECOVIRIMAT OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/21/1600/001 20220107 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TPOXX (Tecovirimat)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
