TIVICAY Drug Patent Profile
✉ Email this page to a colleague
When do Tivicay patents expire, and what generic alternatives are available?
Tivicay is a drug marketed by Viiv Hlthcare and is included in two NDAs. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and fifty-seven patent family members in thirty-five countries.
The generic ingredient in TIVICAY is dolutegravir sodium. There are seventeen drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the dolutegravir sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Tivicay
Tivicay was eligible for patent challenges on August 12, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 8, 2030. This may change due to patent challenges or generic licensing.
There have been twenty-four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are eight tentative approvals for the generic drug (dolutegravir sodium), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TIVICAY?
- What are the global sales for TIVICAY?
- What is Average Wholesale Price for TIVICAY?
Summary for TIVICAY
| International Patents: | 157 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 135 |
| Clinical Trials: | 32 |
| Patent Applications: | 2,411 |
| Drug Prices: | Drug price information for TIVICAY |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TIVICAY |
| What excipients (inactive ingredients) are in TIVICAY? | TIVICAY excipients list |
| DailyMed Link: | TIVICAY at DailyMed |
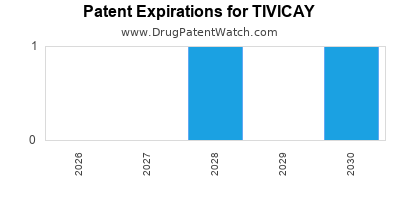
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TIVICAY
Generic Entry Date for TIVICAY*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TIVICAY
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Humanis Saglık Anonim Sirketi | PHASE1 |
| Chelsea and Westminster NHS Foundation Trust | Phase 1 |
| Chelsea and Westminster NHS Foundation Trust | Phase 3 |
Pharmacology for TIVICAY
Paragraph IV (Patent) Challenges for TIVICAY
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TIVICAY | Tablets | dolutegravir sodium | 10 mg, 25 mg and 50 mg | 204790 | 4 | 2017-08-14 |
US Patents and Regulatory Information for TIVICAY
TIVICAY is protected by two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TIVICAY is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | TIVICAY | dolutegravir sodium | TABLET;ORAL | 204790-002 | Jun 9, 2016 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | TIVICAY PD | dolutegravir sodium | TABLET, FOR SUSPENSION;ORAL | 213983-001 | Jun 12, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | TIVICAY | dolutegravir sodium | TABLET;ORAL | 204790-003 | Jun 9, 2016 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | TIVICAY PD | dolutegravir sodium | TABLET, FOR SUSPENSION;ORAL | 213983-001 | Jun 12, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | TIVICAY | dolutegravir sodium | TABLET;ORAL | 204790-002 | Jun 9, 2016 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | TIVICAY | dolutegravir sodium | TABLET;ORAL | 204790-003 | Jun 9, 2016 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for TIVICAY
When does loss-of-exclusivity occur for TIVICAY?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09325128
Estimated Expiration: ⤷ Get Started Free
Patent: 14277831
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0923217
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 44019
Estimated Expiration: ⤷ Get Started Free
Patent: 55957
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2245182
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 76080
Estimated Expiration: ⤷ Get Started Free
Patent: 10603
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 43626
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 86478
Estimated Expiration: ⤷ Get Started Free
Patent: 48595
Estimated Expiration: ⤷ Get Started Free
Patent: 30891
Estimated Expiration: ⤷ Get Started Free
Patent: 12131791
Patent: SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND THEIR INTERMEDIATES
Estimated Expiration: ⤷ Get Started Free
Patent: 12511573
Estimated Expiration: ⤷ Get Started Free
Patent: 16041727
Patent: カルバモイルピリドンHIVインテグラーゼ阻害剤及びそれらの中間体の合成 (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND THEIR INTERMEDIATES)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1942
Patent: SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES.)
Estimated Expiration: ⤷ Get Started Free
Patent: 3683
Patent: SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 11006241
Patent: SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES.)
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 27451
Patent: СИНТЕЗ КАРБАМОИЛПИРИДОНОВЫХ ИНГИБИТОРОВ ИНТЕГРАЗЫ ВИЧ И ПРОМЕЖУТОЧНЫХ СОЕДИНЕНИЙ (SYNTHESIS OF CARBAMOYL PYRIDONE INHIBITORS OF HIV INTEGRASE AND INTERMEDIATE COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 38923
Patent: Синтез карбамоилпиридоновых ингибиторов интегразы ВИЧ и промежуточных соединений (SYNTHESIS OF CARBAMOIL-PYRIDONE INHIBITORS OF HIV INTEGRASE AND INTERMEDIATE COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 11121785
Patent: СИНТЕЗ КАРБАМОИЛПИРИДОНОВЫХ ИНГИБИТОРОВ ИНТЕГРАЗЫ ВИЧ И ПРОМЕЖУТОЧНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Get Started Free
Patent: 13153004
Patent: СИНТЕЗ КАРБАМОИЛПИРИДОНОВЫХ ИНГИБИТОРОВ ИНТЕГРАЗЫ ВИЧ И ПРОМЕЖУТОЧНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 1308
Patent: SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1733625
Estimated Expiration: ⤷ Get Started Free
Patent: 1847887
Estimated Expiration: ⤷ Get Started Free
Patent: 110094336
Patent: SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES
Estimated Expiration: ⤷ Get Started Free
Patent: 170038116
Patent: 카르바모일피리돈 HIV 인테그라제 억제제 및 중간체의 합성 (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 41765
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 83947
Estimated Expiration: ⤷ Get Started Free
Patent: 1030010
Patent: Synthesis of carbamoylpyridone HIV integrase inhibitors and intermediates
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TIVICAY around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Brazil | PI0610030 | composto, processo para a preparação de um composto, método de tratamento de uma infecção por hiv em um humano, uso de um composto, e, composição farmacêutica | ⤷ Get Started Free |
| South Korea | 101848819 | ⤷ Get Started Free | |
| European Patent Office | 1852434 | DÉRIVÉ DE CARBAMOYLPYRIDONE BICYCLIQUE AYANT UNE ACTIVITÉ D'INHIBITION DE LA VIH INTÉGRASE (BICYCLIC CARBAMOYLPYRIDONE DERIVATIVE HAVING HIV INTEGRASE INHIBITING ACTIVITY) | ⤷ Get Started Free |
| European Patent Office | 3284520 | DÉRIVÉ DE CARBAMOYLPYRIDONE POLYCYCLIQUE DOTÉ D'UNE ACTIVITÉ INHIBITRICE DE L'INTÉGRASE DU VIH (POLYCYCLIC CARBAMOYLPYRIDONE DERIVATIVE HAVING HIV INTEGRASE INHIBITORY ACTIVITY) | ⤷ Get Started Free |
| Singapore | 171308 | SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES | ⤷ Get Started Free |
| South Korea | 20130133061 | POLYCYCLIC CARBAMOYLPYRIDONE DERIVATIVE HAVING HIV INTEGRASE INHIBITORY ACTIVITY | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TIVICAY
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1874117 | 14C0041 | France | ⤷ Get Started Free | PRODUCT NAME: DOLUTEGRAVIR ET SES SELS OU SOLVATES PHARMACEUTIQUEMENT ACCEPTABLES,NOTAMMENT LE DOLUTEGRAVIR SODIQUE.; REGISTRATION NO/DATE: EU/1/13/892/001-002 20140121 |
| 2465580 | PA2021512 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: KABOTEGRAVIRAS; REGISTRATION NO/DATE: EU/1/20/1481 20201217 |
| 3494972 | LUC00346 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: DOLUTEGRAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE, Y COMPRIS LE DOLUTEGRAVIR SODIQUE, ET LAMIVUDINE; AUTHORISATION NUMBER AND DATE: EU/1/19/1370 20190703 |
| 2465580 | 21C1023 | France | ⤷ Get Started Free | PRODUCT NAME: CABOTEGRAVIR OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; NAT. REGISTRATION NO/DATE: EU/1/20/1481 20201221; FIRST REGISTRATION: - EU/1/20/1481 20201221 |
| 1874117 | 517 | Finland | ⤷ Get Started Free | |
| 3494972 | C202430022 | Spain | ⤷ Get Started Free | PRODUCT NAME: DOLUTEGRAVIR O UNA SAL FARMACEUTICAMENTE ACEPTABLE, INCLUIDO DOLUTEGRAVIR SODICO Y LAMIVUDINA; NATIONAL AUTHORISATION NUMBER: EU/1/19/1370; DATE OF AUTHORISATION: 20190701; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/19/1370; DATE OF FIRST AUTHORISATION IN EEA: 20190701 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TIVICAY (Dolutegravir)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
