TIBSOVO Drug Patent Profile
✉ Email this page to a colleague
When do Tibsovo patents expire, and when can generic versions of Tibsovo launch?
Tibsovo is a drug marketed by Servier and is included in one NDA. There are ten patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and seventy-six patent family members in forty-four countries.
The generic ingredient in TIBSOVO is ivosidenib. One supplier is listed for this compound. Additional details are available on the ivosidenib profile page.
DrugPatentWatch® Generic Entry Outlook for Tibsovo
Tibsovo was eligible for patent challenges on July 20, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 13, 2035. This may change due to patent challenges or generic licensing.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TIBSOVO?
- What are the global sales for TIBSOVO?
- What is Average Wholesale Price for TIBSOVO?
Summary for TIBSOVO
| International Patents: | 176 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 63 |
| Clinical Trials: | 13 |
| Patent Applications: | 907 |
| Drug Prices: | Drug price information for TIBSOVO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TIBSOVO |
| What excipients (inactive ingredients) are in TIBSOVO? | TIBSOVO excipients list |
| DailyMed Link: | TIBSOVO at DailyMed |
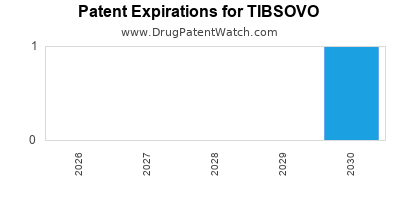

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TIBSOVO
Generic Entry Date for TIBSOVO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TIBSOVO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Pierre Fabre Medicament | Phase 3 |
| Cancer Research UK & UCL Cancer Trials Centre | Phase 3 |
| Accord Healthcare, Inc. | Phase 3 |
Pharmacology for TIBSOVO
Paragraph IV (Patent) Challenges for TIBSOVO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TIBSOVO | Tablets | ivosidenib | 250 mg | 211192 | 1 | 2022-07-20 |
US Patents and Regulatory Information for TIBSOVO
TIBSOVO is protected by thirty-one US patents and six FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TIBSOVO is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,968,595.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | 10,653,710 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | 9,850,277 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | 10,799,490 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Servier | TIBSOVO | ivosidenib | TABLET;ORAL | 211192-001 | Jul 20, 2018 | RX | Yes | Yes | 10,717,764 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for TIBSOVO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Les Laboratoires Servier | Tibsovo | ivosidenib | EMEA/H/C/005936Tibsovo in combination with azacitidine is indicated for the treatment of adult patients with newly diagnosed acute myeloid leukaemia (AML) with an isocitrate dehydrogenase-1 (IDH1) R132 mutation who are not eligible to receive standard induction chemotherapy (see section 5.1).Tibsovo monotherapy is indicated for the treatment of adult patients with locally advanced or metastatic cholangiocarcinoma with an IDH1 R132 mutation who were previously treated by at least one prior line of systemic therapy. | Authorised | no | no | yes | 2023-05-04 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TIBSOVO
When does loss-of-exclusivity occur for TIBSOVO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 15229214
Patent: Pharmaceutical compositions of therapeutically active compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 19246824
Patent: Pharmaceutical compositions of therapeutically active compounds and their methods of use
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2016021232
Patent: composições farmacêuticas de compostos terapeuticamente ativos
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 42072
Patent: COMPOSITIONS PHARMACEUTIQUES ET UTILISATION DE (S)-1-(2-CHLOROPHENYLE)-2-((3,3-DI FLUOROCYCLOBUTYLE)AMINO)-2-OXOETHYLE)-1-(4-CYANOPYRIDIN-2-YL)-N-(5-FLUOROPYRIDIN-3-YL)-5-OXOPYRROLIDINE-2-CARBOXAMIDE (PHARMACEUTICAL COMPOSITIONS AND USE OF (S)-1-(2-CHLOROPHENYL)-2-((3,3-DIFLUOROCYCLOBUTYL)AMINO)-2-OXOETHYL)-1-( 4-CYANOPYRIDIN-2-YL)-N-( 5-FLUOROPYRIDIN-3-YL)-5-OXOPYRROLIDINE-2-CARBOXAMIDE)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 6255498
Patent: 治疗活性化合物的药物组合物 (Pharmaceutical compositions of therapeutically active compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 2159391
Patent: 治疗活性化合物的药物组合物 (Pharmaceutical compositions of therapeutically active compounds)
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6325
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ НА ОСНОВЕ ТВЕРДОЙ ДИСПЕРСИИ ИНГИБИТОРА IDH1 (PHARMACEUTICAL COMPOSITION COMPRISING SOLID DISPERSION OF IDH1 INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 1691845
Patent: ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ ТЕРАПЕВТИЧЕСКИ АКТИВНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 16492
Patent: COMPOSITIONS PHARMACEUTIQUES DE COMPOSÉS THÉRAPEUTIQUEMENT ACTIFS (PHARMACEUTICAL COMPOSITIONS OF THERAPEUTICALLY ACTIVE COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7722
Patent: Oral pharmaceutical compositions comprising a solid dispersion of (s)-n-((s)-1-(2-chlorophenyl)-2-((3,3-difluorocyclobutyl)amino)-2-oxoethyl)-1-(4-cyanopyridin-2-yl)-n-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide and hpmcas or hpmc and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 3319
Patent: צורה גבישית של (s) - n-1-(2-כלורופניל)-2-((3,3-דיפלורוציקלוביוטיל)אמינו)-2-אוקסואטיל)-1-(4-ציאנופירידין-2-יל)-n-(5-פלורופירידין-3-יל)-5-אוקסופירולידין-2-קארבוקסאמיד, תכשירים המכילים אותה, שיטות להכנתה, ושימושים בה (Crystalline form of (s)-n-((s)-1-(2-chlorophenyl)-2-((3,3-difluorocyclobutyl)amino)-2-oxoethyl)-1-(4-cyanopyridin-2-yl)-n-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide, compositions comprising same, processes to produce same and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 64796
Estimated Expiration: ⤷ Get Started Free
Patent: 17508805
Patent: 治療活性化合物の医薬組成物
Estimated Expiration: ⤷ Get Started Free
Patent: 20007313
Patent: 治療活性化合物の医薬組成物 (PHARMACEUTICAL COMPOSITIONS OF THERAPEUTICALLY ACTIVE COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 21191785
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 16011810
Estimated Expiration: ⤷ Get Started Free
Patent: 20011104
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 726
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3859
Estimated Expiration: ⤷ Get Started Free
Patent: 2612
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 016501790
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2400737
Estimated Expiration: ⤷ Get Started Free
Patent: 160124914
Estimated Expiration: ⤷ Get Started Free
Patent: 220070066
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1211
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TIBSOVO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Nicaragua | 201400078 | DERIVADOS DE LACTAMAS ÚTILES COMO INHIBIDORES MUTANTES DE IDH1 | ⤷ Get Started Free |
| Canada | 2942072 | ⤷ Get Started Free | |
| Serbia | 62829 | KOMBINOVANA TERAPIJA ZA LEČENJE MALIGNITETA (COMBINATION THERAPY FOR TREATING MALIGNANCIES) | ⤷ Get Started Free |
| South Africa | 202308496 | ⤷ Get Started Free | |
| Japan | 2015044862 | 細胞増殖関連疾患のための方法および組成物 (METHODS AND COMPOSITIONS FOR CELL-PROLIFERATION-RELATED DISORDERS) | ⤷ Get Started Free |
| Japan | 6805220 | ⤷ Get Started Free | |
| South Korea | 20250156852 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TIBSOVO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2804851 | 2023C/534 | Belgium | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIB OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT, TAUTOMEER, ISOTOPOLOOG OF HYDRAAT DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/23/1728 20230508 |
| 2804851 | PA2023529,C2804851 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIBAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA, TAUTOMERAS, IZOTOPOLOGAS ARBA HIDRATAS; REGISTRATION NO/DATE: EU/1/23/1728 20230504 |
| 2804851 | CA 2023 00025 | Denmark | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIB ELLER ET FARMACEUTISK ACCEPTABELT SALT, TAUTOMER, ISOTOPOLOG ELLER HYDRAT DERAF; REG. NO/DATE: EU/1/23/1728 20230508 |
| 2804851 | 2390027-7 | Sweden | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIB OR A PHARMACEUTICALLY ACCEPTABLE SALT, TAUTOMER, ISOTOPOLOGUE OR HYDRATE THEREOF; REG. NO/DATE: EU/1/23/1728 20230508 |
| 2804851 | LUC00315 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIB OR A PHARMACEUTICALLY ACCEPTABLE SALT, TAUTOMER, ISOTOPOLOGUE OR HYDRATE THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/23/1728 20230508 |
| 2804851 | C202330033 | Spain | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIB O UNA SAL, TAUTOMERO, ISOTOPOLOGO O HIDRATO FARMACEUTICAMENTE ACEPTABLE DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/23/1728; DATE OF AUTHORISATION: 20230504; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/23/1728; DATE OF FIRST AUTHORISATION IN EEA: 20230504 |
| 2804851 | 301243 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: IVOSIDENIB OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT, TAUTOMEER, ISOTOPOLOOG OF HYDRAAT DAARVAN; REGISTRATION NO/DATE: EU/1/23/1728 20230508 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TIBSOVO (Ivosidenib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
