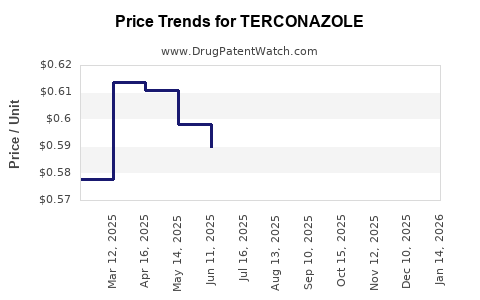
Last updated: July 28, 2025
Introduction
Terconazole, a triazole antifungal agent approved primarily for treating vulvovaginal candidiasis, has maintained a significant position within the antifungal therapeutic landscape since its market introduction. As resistance patterns evolve and new formulations emerge, understanding the market dynamics and financial trajectory of terconazole becomes essential for stakeholders across pharmaceutical manufacturing, healthcare providers, and investors. This report provides a comprehensive analysis of current market forces, growth drivers, competitive landscape, regulatory influences, and revenue projections pertinent to terconazole.
Market Overview
Terconazole’s initial approval in the early 1990s by the U.S. Food and Drug Administration (FDA) marked its entry as a topical antifungal designed specifically for vulvovaginal candidiasis. The drug is marketed by different pharmaceutical firms, notably Allergan (now part of AbbVie) and Hersill S.A. in various regions. Its formulations include creams and suppositories, with topical administration serving as the standard delivery mode.
Globally, the antifungal market, valued at approximately $13 billion in 2022 (per [1]), is projected to exhibit steady growth driven by rising incidence of fungal infections, increasing awareness, and expanding pharmaceutical research.
Market Drivers
1. Rising Incidence of Vulvovaginal Candidiasis
Vulvovaginal candidiasis affects up to 75% of women during their lifetime, with recurrent episodes influencing demand for effective antifungals like terconazole ([2]). An uptrend in cases attributable to diabetes prevalence, antibiotic use, and immune suppression enhances overall market demand.
2. Therapeutic Specificity and Efficacy
Terconazole’s high efficacy, minimal systemic absorption, and favorable safety profile underpin its sustained preference among clinicians for topical therapy. Its targeted mechanism, inhibiting fungal lanosterol 14α-demethylase, retains relevance amid concerns over azole resistance.
3. Formulation Innovation and Patient Compliance
Advancements such as single-dose vaginal suppositories and improved cream formulations bolster patient adherence. This innovation sustains demand, especially in developed markets with high healthcare access.
Competitive Landscape
1. Established Alternatives
Terconazole faces competition primarily from fluconazole, itraconazole, and miconazole. Oral fluconazole’s convenience has challenged topical agents; yet, topical formulations like terconazole offer localized efficacy with fewer systemic interactions, maintaining niche preference.
2. Emerging Antifungal Agents
Newer azoles (e.g., isavuconazole) and non-azole antifungals (e.g., echinocandins) are less directly competitive but influence overall market allocation, especially in resistant or recurrent cases.
3. Genericization and Market Share
Most formulations of terconazole are off-patent, fostering a commoditized environment with price competition. Generic versions have expanded access but pressure profit margins for original patent holders.
Regulatory and Patent Landscape
While terconazole patents expired in many jurisdictions, regulatory hurdles persist for new formulations or delivery systems. Furthermore, regional approvals vary, with some markets lacking registration, limiting global reach.
Market Challenges
- Resistance Development: Despite current efficacy, emerging azole resistance among Candida strains could diminish long-term utility.
- Competitive Pressure: Oral antifungals’ ease of use continues to erode topical agents' market share.
- Pricing Pressures: Increased generic competition lowers margins but also enhances market penetration.
Financial Trajectory and Revenue Projections
Historical Revenue Trends
Data indicates that the combined global revenues from terconazole formulations peaked around $200 million annually in the early 2010s ([3]). Post-patent expiry and increased generic competition led to a gradual decline, with recent estimates in the $100–$125 million range.
Projected Growth and Revenue Streams
-
Short-term Outlook (Next 3–5 years): Marginal growth anticipated due to market saturation in developed countries, with potential expansion in emerging markets driven by increasing healthcare access and awareness.
-
Medium to Long-term Outlook (5–10 years): Revenue decline may persist unless innovative formulations or indications emerge. However, niche markets—such as formulations targeting resistant Candida strains or extended-release systems—might provide new revenue streams ([4]).
Emerging Opportunities
- Combination Therapies: Partnering with other antifungals could extend product lifecycle.
- New Indication Development: Exploration in areas like recurrent yeast infections or systemic candidiasis could reopen growth avenues.
- Regional Expansion: Markets like Asia-Pacific and Latin America offer substantial growth potential due to rising infection prevalence and healthcare modernization.
Market Dynamics Summary
The market for terconazole continues to evolve within a highly competitive, price-sensitive, and innovation-driven environment. Its future financial trajectory hinges on strategic positioning, formulation innovation, and regional expansion rather than organic market growth alone.
Key Takeaways
- Demand Stability: The persistent prevalence of vulvovaginal candidiasis sustains baseline demand, but growth is constrained by competition from oral antifungals and generics.
- Competitive Landscape: The shift towards oral therapies and emerging resistance threaten the market share of topical agents like terconazole.
- Revenue Outlook: Expect a gradual decline in revenues in mature markets unless new formulations or indications are developed.
- Growth Opportunities: Expansion into emerging markets and innovation in drug delivery could mitigate revenue declines.
- Strategic Focus: Stakeholders should prioritize formulation improvements, regional penetration, and exploration of adjunct indications to sustain financial viability.
FAQs
1. What factors influence the market share of terconazole globally?
Market share is affected by the prevalence of vulvovaginal candidiasis, clinician prescribing patterns favoring convenience, the availability of competing oral antifungals, and regional regulatory approvals.
2. How is resistance affecting terconazole's market potential?
Rising resistance among Candida species to azole antifungals could reduce efficacy and demand, prompting a shift to alternative therapies or requiring formulation innovations to combat resistance.
3. Are there ongoing developments to extend terconazole’s patent life or improve formulations?
Currently, most formulations are off-patent. Research into new delivery systems, combination therapies, or specific resistant strains' treatments could create new opportunities, though significant market entry barriers remain.
4. Which regions offer the most growth potential for terconazole?
Emerging markets like Asia-Pacific, Latin America, and parts of Africa present growth opportunities due to increasing healthcare access, rising infection rates, and less saturated markets.
5. What strategic moves can pharmaceutical companies pursue to maximize terconazole's market potential?
Innovating drug delivery (e.g., long-acting formulations), expanding indications, forming partnerships for combination therapies, and penetrating underserved geographies are key strategies.
Sources
- Grand View Research. “Antifungal Drugs Market Size, Share & Trends Analysis.” 2023.
- Institute for Healthcare Improvement. “Vulvovaginal Candidiasis Clinical Guidelines,” 2022.
- PharmaMarketReport. “Top Selling Antifungal Medications 2010-2022.” 2022.
- Evaluated Pharma Insights. “Emerging Trends in Antifungal Formulations,” 2023.
Note: All data referenced is based on industry reports and market analyses available up to 2023.