TECHNIVIE Drug Patent Profile
✉ Email this page to a colleague
When do Technivie patents expire, and when can generic versions of Technivie launch?
Technivie is a drug marketed by Abbvie and is included in one NDA. There are eight patents protecting this drug.
This drug has four hundred and fifty-seven patent family members in forty-eight countries.
The generic ingredient in TECHNIVIE is ombitasvir; paritaprevir; ritonavir. Additional details are available on the ombitasvir; paritaprevir; ritonavir profile page.
DrugPatentWatch® Generic Entry Outlook for Technivie
Technivie was eligible for patent challenges on December 19, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 13, 2032. This may change due to patent challenges or generic licensing.
There have been twelve patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TECHNIVIE?
- What are the global sales for TECHNIVIE?
- What is Average Wholesale Price for TECHNIVIE?
Summary for TECHNIVIE
| International Patents: | 457 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 5 |
| Drug Prices: | Drug price information for TECHNIVIE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TECHNIVIE |
| DailyMed Link: | TECHNIVIE at DailyMed |
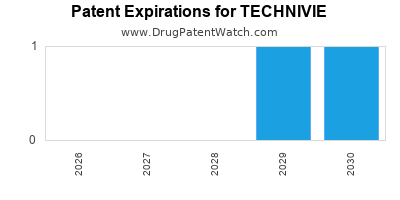

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TECHNIVIE
Generic Entry Date for TECHNIVIE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TECHNIVIE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| AbbVie | Phase 3 |
| AbbVie | Phase 2 |
US Patents and Regulatory Information for TECHNIVIE
TECHNIVIE is protected by eight US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TECHNIVIE is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for TECHNIVIE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | TECHNIVIE | ombitasvir; paritaprevir; ritonavir | TABLET;ORAL | 207931-001 | Jul 24, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Abbvie | TECHNIVIE | ombitasvir; paritaprevir; ritonavir | TABLET;ORAL | 207931-001 | Jul 24, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Abbvie | TECHNIVIE | ombitasvir; paritaprevir; ritonavir | TABLET;ORAL | 207931-001 | Jul 24, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Abbvie | TECHNIVIE | ombitasvir; paritaprevir; ritonavir | TABLET;ORAL | 207931-001 | Jul 24, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Abbvie | TECHNIVIE | ombitasvir; paritaprevir; ritonavir | TABLET;ORAL | 207931-001 | Jul 24, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Abbvie | TECHNIVIE | ombitasvir; paritaprevir; ritonavir | TABLET;ORAL | 207931-001 | Jul 24, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for TECHNIVIE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AbbVie Deutschland GmbH Co. KG | Viekirax | ombitasvir, paritaprevir, ritonavir | EMEA/H/C/003839Viekirax is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adults.For hepatitis C virus (HCV) genotype specific activity. | Authorised | no | no | no | 2015-01-14 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TECHNIVIE
When does loss-of-exclusivity occur for TECHNIVIE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7060
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 3240
Patent: COMPOSICIONES SOLIDAS PARA TRATAMIENTO DE INFECCIONES DEL HCV (HEPATITIS C)
Estimated Expiration: ⤷ Get Started Free
Patent: 3398
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 2505
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 4816
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10258769
Patent: Anti-viral compounds to treat HCV infection
Estimated Expiration: ⤷ Get Started Free
Patent: 11264823
Patent: Solid compositions
Estimated Expiration: ⤷ Get Started Free
Patent: 11316506
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1004894
Patent: compostos antivirais
Estimated Expiration: ⤷ Get Started Free
Patent: 2012031500
Estimated Expiration: ⤷ Get Started Free
Patent: 2013005701
Patent: compostos antivirais
Estimated Expiration: ⤷ Get Started Free
Patent: 2012031500
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 37601
Patent: COMPOSES ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 02180
Patent: COMPOSITIONS SOLIDES (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 07847
Patent: COMPOSES ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19894
Patent: COMPOSES ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 28495
Patent: COMPOSES ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 47910
Patent: COMPOSES ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 38547
Patent: COMPOSES ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 97170
Patent: DERIVES ANTI-VIRAUX DE TETRAHYDROFURANE (ANTI-VIRAL TETRAHYDROFURANE DERIVATIVES)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11000689
Patent: Compuesto derivado de ((4-terbutilfenil)pirrolidin-2,5-diil)bis(4,1-fenilen)sustituido, inhibidor de la replicacion del vhc; composicion farmaceutica; util en el tratamiento de una infeccion por vhc.
Estimated Expiration: ⤷ Get Started Free
Patent: 12003470
Patent: Una composicion solida que comprende 1) un compuesto inhibidor del hcv de formula definida, o una sal farmaceuticamente aceptable de este, en una forma amorfa, 2) un polimero hidrofilico, y opcionalmente 3) un agente tensoactivo; su proceso de preparacion, util para tratar una infeccion del hcv.
Estimated Expiration: ⤷ Get Started Free
Patent: 13000970
Patent: Compuestos derivados de heterociclos nitrogenados sustituidos; sus composiciones farmaceuticas, y uso como inhiibidores de la replicacion del hcv para el tratamiento de la hepatitis c.
Estimated Expiration: ⤷ Get Started Free
Patent: 13002299
Patent: Compuestos derivados de heterociclos, inhibidores de la replicacion del virus de la hepatitis c; composicion farmaceutica que los comprende; util en el tratamiento de una infeccion por vhc. (divisional solicitud 689-2011).
Estimated Expiration: ⤷ Get Started Free
Patent: 14000059
Patent: Compuestos derivados de heterociclos nitrogenados sustituidos; proceso de preparacion; compuesto intermediario; composiciones farmaceuticas; utiles como inhiibidores de la replicacion del hcv para tratar la hepatitis c. (div. sol. 970-13)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2333772
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 3153988
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 3172620
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 3209686
Patent: Solid compositions
Estimated Expiration: ⤷ Get Started Free
Patent: 3354808
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 3596941
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 3819459
Patent: Anti-Viral Compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 3819537
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 4193729
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 6986861
Patent: 抗病毒化合物 (Anti-viral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 8350016
Patent: 抗病毒四氢呋喃衍生物 (Anti-viral tetrahydrofurane derivatives)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 40538
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 60490
Patent: Composiciones solidas
Estimated Expiration: ⤷ Get Started Free
Patent: 61348
Patent: Compuestos antivirales
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 120650
Patent: COMPOSICIONES SÓLIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 130170
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 140021
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0130671
Estimated Expiration: ⤷ Get Started Free
Patent: 0150017
Estimated Expiration: ⤷ Get Started Free
Patent: 0150926
Estimated Expiration: ⤷ Get Started Free
Patent: 0151389
Estimated Expiration: ⤷ Get Started Free
Patent: 0160408
Estimated Expiration: ⤷ Get Started Free
Patent: 0181658
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 14356
Estimated Expiration: ⤷ Get Started Free
Patent: 16060
Estimated Expiration: ⤷ Get Started Free
Patent: 16748
Estimated Expiration: ⤷ Get Started Free
Patent: 17188
Estimated Expiration: ⤷ Get Started Free
Patent: 17456
Estimated Expiration: ⤷ Get Started Free
Patent: 22267
Estimated Expiration: ⤷ Get Started Free
Patent: 15012
Estimated Expiration: ⤷ Get Started Free
Patent: 17031
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 68890
Estimated Expiration: ⤷ Get Started Free
Patent: 55376
Estimated Expiration: ⤷ Get Started Free
Patent: 79854
Estimated Expiration: ⤷ Get Started Free
Patent: 28481
Estimated Expiration: ⤷ Get Started Free
Patent: 92346
Estimated Expiration: ⤷ Get Started Free
Patent: 54892
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 011000087
Patent: Compuestos eficaces para inhibir la replicación del virus de la hepatitis c("HCV")
Estimated Expiration: ⤷ Get Started Free
Patent: 012000307
Patent: COMPOSICIONES SOLIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 013000078
Patent: COMPUESTO DERIVADO DE HETEROCICLOS NITROGENADOS SUSTITUIDOS, EFICAZ COMO INHIBIDOR DE LA REPLICACIÓN DEL VIRUS DE LA HEPATITIS C, COMPOSICIÓN QUE LO COMPRENDE Y SU USO
Estimated Expiration: ⤷ Get Started Free
Patent: 013000185
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 11010937
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 13010937
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 13012382
Patent: COMPOSICIONES SÓLIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 13012622
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 14012622
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 17064986
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0031
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 3570
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 4100
Patent: МЕТИЛ {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-ДИФТОР-4-[4-(4-ФТОРФЕНИЛ)ПИПЕРИДИН-1-ИЛ]ФЕНИЛ}-5-(6-ФТОР-2-{(2S)-1-[N-(МЕТОКСИКАРБОНИЛ)-О-МЕТИЛ-L-ТРЕОНИЛ]ПИРРОЛИДИН-2-ИЛ}-1H-БЕНЗИМИДАЗОЛ-5-ИЛ)ПИРРОЛИДИН-2-ИЛ]-6-ФТОР-1H-БЕНЗИМИДАЗОЛ-2-ИЛ}ПИРРОЛИДИН-2-ИЛ]-3-МЕТОКСИ-1-ОКСОБУТАН-2-ИЛ}КАРБАМАТ, ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, ВКЛЮЧАЮЩАЯ ЕГО, И СПОСОБ ЛЕЧЕНИЯ ИНФЕКЦИИ ГЕПАТИТА C (METHYL {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-DIFLUORO-4-[4-(4-FLUOROPHENYL)PIPERIDIN-1-YL]PHENYL}-5-(6-FLUORO-2-{(2S)-1-[N-(METHOXYCARBONYL)-O-METHYL-L-THREONYL]PYRROLIDIN-2-yl}-1H-BENZIMIDAZOL-5-YL)PYRROLIDIN-2-YL]-6-FLUORO-1H-BENZIMIDAZOL-2-YL}PYRROLIDIN-1-YL]-3-METHOXY-1-OXOBUTAN-2-YL}CARBAMATE, PHARMACEUTICAL COMPOSITION COMPRISING SAME, AND METHOD OF TREATING HCV INFECTION)
Estimated Expiration: ⤷ Get Started Free
Patent: 4538
Patent: ТВЕРДАЯ ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, СОДЕРЖАЩАЯ СОЕДИНЕНИЕ С АНТИ-ВГС (HCV) АКТИВНОСТЬЮ (SOLID PHARMACEUTICAL COMPOSITION COMPRISING A COMPOUND WITH ANTI-HCV ACTIVITY)
Estimated Expiration: ⤷ Get Started Free
Patent: 6848
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ (ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 3332
Patent: СПОСОБ ПОЛУЧЕНИЯ МЕТИЛ {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-ДИФТОР-4-[4-(4-ФТОРФЕНИЛ)ПИПЕРИДИН-1-ИЛ]ФЕНИЛ}-5-(6-ФТОР-2-{(2S)-1-[N-(МЕТОКСИКАРБОНИЛ)-О-МЕТИЛ-L-ТРЕОНИЛ]ПИРРОЛИДИН-2-ИЛ}-1Н-БЕНЗИМИДАЗОЛ-5-ИЛ)ПИРРОЛИДИН-2-ИЛ]-6-ФТОР-1Н-БЕНЗИМИДАЗОЛ-2-ИЛ}ПИРРОЛИДИН-1-ИЛ]-3-МЕТОКСИ-1-ОКСОБУТАН-2-ИЛ} КАРБАМАТА (PROCESS FOR PREPARING METHYL{(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-DIFLUORO-4-[4-(4-FLUOROPHENYL)PIPERIDINE-1-YL]PHENYL}-5-(6-FLUORO-2-{(2S)-1-[N-(METHOXYCARBONYL)-O-METHYL-L-THREONYL]PYRROLIDINE-2-YL}-1H-BENZIMIDAZOLE-5-YL)pyrrolidinePYRROLIDINE-2-YL]-6-FLUORO-1H-BENZIMIDAZOLE-2-YL}PYRROLIDINE-1-YL]-3-METHOXY-1-OXOBUTANE-2-YL}CARBAMATE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1170401
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 1291394
Patent: ТВЕРДЫЕ КОМПОЗИЦИИ
Estimated Expiration: ⤷ Get Started Free
Patent: 1300495
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 1301158
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 1390538
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Patent: 1400115
Patent: ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 37781
Patent: COMPOSÉS ANTIVIRAUX POUR LE TRAITEMENT D'INFECTIONS HCV (ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION)
Estimated Expiration: ⤷ Get Started Free
Patent: 68890
Patent: Inhibiteurs de la hépatite C (Hepatitis C virus inhibitors)
Estimated Expiration: ⤷ Get Started Free
Patent: 55376
Patent: Composés hétérocycliques comme inhibiteurs du virus de'l hepatite C (HCV) (Heterocyclic compounds as inhibitors of hepatitis C virus (HCV))
Estimated Expiration: ⤷ Get Started Free
Patent: 79854
Patent: COMPOSITIONS SOLIDES (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 27651
Patent: COMPOSÉS ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 28481
Patent: Dérivés hétérocycliques trisubstitués comme inhibiteurs de la réplication du virus de l'hépatite C HCV (Trisubstituted heterocycles as replication inhibitors of hepatitis C virus HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 51885
Patent: COMPOSÉS ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 78334
Patent: COMPOSÉS ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 92346
Patent: Dérivé antiviraux de 2,5-dibenzimidazol-5-yl-1-phényl-pyrrolidine (An antiviral 1-phenyl-2,5-dibenzimidazol-5-yl-pyrrolidine derivative)
Estimated Expiration: ⤷ Get Started Free
Patent: 92726
Patent: Composés antiviraux (Anti-viral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 53531
Patent: Composés antiviraux (Antiviral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 54892
Patent: COMPOSITIONS SOLIDES (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 44642
Patent: DÉRIVÉS ANTI-VIRAUX DE TÉTRAHYDROFURANE (ANTI-VIRAL TETRAHYDROFURANE DERIVATIVES)
Estimated Expiration: ⤷ Get Started Free
Patent: 38106
Patent: COMPOSÉS ANTIVIRAUX (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 56318
Patent: COMPOSITIONS SOLIDES (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1040
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1100074
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 1200332
Patent: COMPOSICIONES SÒLIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 1300093
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 1300093A
Patent: COMPUESTOS ANTIVIRALES (SOLICITUD DIVISIONAL FRACCIONARIA DE LA SOLICITUD A-2013-00093)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 52620
Patent: 治療 感染的抗病毒化合物 (ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 61245
Patent: HEPATITIS C VIRUS INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Patent: 70739
Patent: 作為丙型肝炎病毒 抑制劑的雜環化合物 (HETEROCYCLIC COMPOUNDS AS INHIBITORS OF HEPATITIS C VIRUS (HCV) (HCV))
Estimated Expiration: ⤷ Get Started Free
Patent: 84068
Patent: 固體組合物 (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 86415
Patent: 作為丙型肝炎病毒 的複製抑制劑的三取代雜環 (TRISUBSTITUTED HETEROCYCLES AS REPLICATION INHIBITORS OF HEPATITIS C VIRUS HCV HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 88717
Patent: 種抗病毒 -苯基- -二苯並咪唑- -基-吡咯烷衍生物 (AN ANTIVIRAL 1-PHENYL-2,5-DIBENZIMIDAZOL-5-YL-PYRROLIDINE DERIVATIVE 1--25--5--)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 25758
Estimated Expiration: ⤷ Get Started Free
Patent: 26832
Estimated Expiration: ⤷ Get Started Free
Patent: 28825
Estimated Expiration: ⤷ Get Started Free
Patent: 39719
Estimated Expiration: ⤷ Get Started Free
Patent: 500019
Estimated Expiration: ⤷ Get Started Free
Patent: 700040
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1792
Patent: דימתיל (2s,2's)-1,'1-((2s,2's)-2,'2-(4,'4-((2s,5s)-1-(4-טרט-בוטילפניל)פירולידין-5,2-דיאיל)ביס(1,4 פנילן))ביס (אזאנדיאיל)ביס(אוקסומתילן)ביס(פירולידין-1,2-דיאיל))ביס(3- מתיל-1-אוקסובוטן-1,2-דיאיל)דיקארבמאט והרכב רוקחי המכיל אותו (Dimethyl (2s, 2's)-1, 1'-((2s,2's)-2,2'-(4,4' -((2s,5s)-1 -(4-tert-butylphenyl) pyrrolidine-2,5 -diyl) bis (4,1 phenylene)) bis (azanediyl) bis (oxomethylene) bis (pyrrolidine-2,1-diyl)) bis (3-methyl-1-oxobutane-2,1 -diyl) dicarbamate and a pharmaceutical composition comprising same)
Estimated Expiration: ⤷ Get Started Free
Patent: 3535
Patent: הרכבים תרופתיים מוצקים (Solid pharmaceutical compositions)
Estimated Expiration: ⤷ Get Started Free
Patent: 5010
Patent: מתיל (2s,3r)-1-((1s)-2-(-2-(-5-((2r,3r)-1-(3,5,דיפלואורו-4-(4-(4-פלואורופניל)פיפרידינ-1-איל(פניל)-5-(6-פלואורו-2-((2s)-1-(n-(מתוקסיקרבוניל)-o-מתיל-l-תראוניל)פירולידינ-1-איל)-1י-בנזואימידזול-5-איל)פירולידין-2-איל)-6-פלואורו-1h-בנזאימידזול-2-איל)פירולידינ-1-איל)-3-מתוקסי-1-אוקסובוטאנ-2-איל)קרבאמט והרכב רוקחי המכיל אותו (Methyl {(2s,3r)-1-[(2s)-2-{5-[(2r,5r)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}-5-(6-fluoro-2-{(2s)-1-[n-(methoxycarbonyl)-o-methyl-l-threonyl]pyrrolidin-2-yl}-1h-benzimidazol-5-yl)pyrrolidin-2-yl]-6-fluoro-1h-benzimidazol-2-yl}pyrrolidin-1-yl]-3-methoxy-1-oxobutan-5 2-yl}carbamate and a pharmaceutical composition comprising it)
Estimated Expiration: ⤷ Get Started Free
Patent: 9248
Patent: תרכובות נגד נגיפים והרכבים רוקחיים המכילים אותן (Anti-viral compounds and pharmaceutical compositions comprising same)
Estimated Expiration: ⤷ Get Started Free
Patent: 3857
Patent: תרכובות נגד נגיפים (Anti-viral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 4781
Patent: תרכובות נגד נגיפים (Anti-viral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 1206
Patent: תרכובות אנטי-וירליות (Anti-viral compounds)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 30514
Estimated Expiration: ⤷ Get Started Free
Patent: 14356
Estimated Expiration: ⤷ Get Started Free
Patent: 34085
Estimated Expiration: ⤷ Get Started Free
Patent: 06253
Estimated Expiration: ⤷ Get Started Free
Patent: 11838
Estimated Expiration: ⤷ Get Started Free
Patent: 22699
Estimated Expiration: ⤷ Get Started Free
Patent: 59830
Estimated Expiration: ⤷ Get Started Free
Patent: 59736
Estimated Expiration: ⤷ Get Started Free
Patent: 86147
Estimated Expiration: ⤷ Get Started Free
Patent: 43135
Estimated Expiration: ⤷ Get Started Free
Patent: 90202
Estimated Expiration: ⤷ Get Started Free
Patent: 12529534
Estimated Expiration: ⤷ Get Started Free
Patent: 13528225
Estimated Expiration: ⤷ Get Started Free
Patent: 13539791
Estimated Expiration: ⤷ Get Started Free
Patent: 14065710
Patent: ANTI-VIRAL COMPOUNDS FOR TREATING HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Patent: 14144973
Patent: ANTI-VIRAL COMPOUND FOR TREATING HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Patent: 14504296
Estimated Expiration: ⤷ Get Started Free
Patent: 14510063
Estimated Expiration: ⤷ Get Started Free
Patent: 16106075
Patent: 抗ウィルス化合物 (ANTI-VIRUS COMPOUND)
Estimated Expiration: ⤷ Get Started Free
Patent: 16128456
Patent: HCV感染を治療するための抗ウィルス化合物 (ANTI-VIRAL COMPOUNDS FOR TREATING HCV INFECTION)
Estimated Expiration: ⤷ Get Started Free
Patent: 17171680
Patent: HCV感染を治療するための抗ウィルス化合物 (ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION)
Estimated Expiration: ⤷ Get Started Free
Patent: 18065828
Patent: 抗ウィルス化合物 (ANTI-VIRUS COMPOUND)
Estimated Expiration: ⤷ Get Started Free
Patent: 18529671
Patent: 抗ウィルス性テトラヒドロフラン誘導体
Estimated Expiration: ⤷ Get Started Free
Patent: 19194254
Patent: HCV感染を治療するための抗ウィルス化合物 (ANTI-VIRAL COMPOUNDS FOR TREATING HCV INFECTION)
Estimated Expiration: ⤷ Get Started Free
Patent: 20059696
Patent: 抗ウィルス化合物 (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 21035964
Patent: 抗ウィルス化合物 (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 368890
Estimated Expiration: ⤷ Get Started Free
Patent: 692346
Estimated Expiration: ⤷ Get Started Free
Patent: 2017033
Estimated Expiration: ⤷ Get Started Free
Patent: 54892
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0038
Estimated Expiration: ⤷ Get Started Free
Patent: 668
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 4064
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 4607
Patent: SOLID COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 6633
Patent: HETEROCYCLIC COMPOUNDS AS INHIBITORS OF HEPATITIS C VIRUS (HCV)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9989
Patent: COMPUESTOS ANTI-VIRALES. (ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION.)
Estimated Expiration: ⤷ Get Started Free
Patent: 4092
Patent: COMPUESTOS ANTIVIRALES. (ANTI-VIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 6264
Patent: COMPUESTOS ANTIVIRALES. (ANTI-VIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11005673
Patent: COMPUESTOS ANTI-VIRALES PARA TRATAR INFECCION POR VHC. (ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION.)
Estimated Expiration: ⤷ Get Started Free
Patent: 12014384
Patent: COMPOSICIONES SOLIDAS. (SOLID COMPOSITIONS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13004150
Patent: CONPUESTOS ANTIVIRALES. (ANTI-VIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13006951
Patent: COMPUESTOS ANTIVIRALES. (ANTI-VIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13009763
Patent: COMPUESTOS ANTIVIRALES. (ANTI-VIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 18002707
Patent: DERIVADOS DE TETRAHIDROFURANO ANTIVIRALES. (ANTI-VIRAL TETRAHYDROFURANE DERIVATIVES.)
Estimated Expiration: ⤷ Get Started Free
Patent: 20002151
Patent: COMPUESTOS ANTIVIRALES. (ANTI-VIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 201
Patent: PRIPRAVCI U KRUTOM STANJU (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 328
Patent: ANTIVIRUSNI 2, 5-DIBENZIMIDAZOL- 5- IL-1-FENIL- PIROLIDINDERIVAT (An antiviral 1- phenyl- 2,5-dibenzimidazol-5-yl-pyrrolidine derivative)
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 0731
Estimated Expiration: ⤷ Get Started Free
Patent: 0901
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1973
Patent: ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Patent: 5440
Patent: SOLID COMPOSITIONS COMPRISING AN HCV INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 6645
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 5562
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 15012
Estimated Expiration: ⤷ Get Started Free
Patent: 17057
Estimated Expiration: ⤷ Get Started Free
Patent: 21039
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 110679
Patent: DERIVADOS DE (4-TERT-BUTILFENIL)PIRROLIDIN-2,5-DIFENIL COMO INHIBIDORES DEL HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 131036
Patent: COMPOSICIONES SOLIDAS QUE COMPRENDEN COMPUESTOS ANTI-HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 140038
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 140835
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 141083
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012502442
Patent: SOLID COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 013500708
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 015500289
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 68890
Estimated Expiration: ⤷ Get Started Free
Patent: 55376
Estimated Expiration: ⤷ Get Started Free
Patent: 79854
Estimated Expiration: ⤷ Get Started Free
Patent: 28481
Estimated Expiration: ⤷ Get Started Free
Patent: 92346
Estimated Expiration: ⤷ Get Started Free
Patent: 54892
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 68890
Estimated Expiration: ⤷ Get Started Free
Patent: 55376
Estimated Expiration: ⤷ Get Started Free
Patent: 79854
Estimated Expiration: ⤷ Get Started Free
Patent: 92346
Estimated Expiration: ⤷ Get Started Free
Patent: 54892
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500263
Patent: COMPOSIZIONI SOLIDE
Estimated Expiration: ⤷ Get Started Free
Patent: 01800532
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 854
Patent: INHIBITORI HEPATITIS C VIRUSA (HEPATITIS C VIRUS INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Patent: 856
Patent: HETEROCIKLIČNA JEDINJENJA KAO INHIBITORI HEPATITIS C VIRUSA (HETEROCYCLIC COMPOUNDS AS INHIBITORS OF HEPATITIS C VIRUS (HCV))
Estimated Expiration: ⤷ Get Started Free
Patent: 282
Patent: ČVRSTE SMEŠE (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 619
Patent: ANTIVIRUSNI DERIVAT 1-FENIL-2,5-DIBENZIMIDAZOL-5-IL-PIROLIDINA (AN ANTIVIRAL 1-PHENYL-2,5-DIBENZIMIDAZOL-5-YL-PYRROLIDINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Patent: 790
Patent: TRISUPSTITUISANI HETEROCIKLI KAO INHIBITORI REPLIKACIJE VIRUSA HEPATITISA C HCV (TRISUBSTITUTED HETEROCYCLES AS REPLICATION INHIBITORS OF HEPATITIS C VIRUS HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 982
Patent: ČVRSTI SASTAVI (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 1708
Patent: ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Patent: 6251
Patent: SOLID COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 8951
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 201702522U
Patent: ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 68890
Estimated Expiration: ⤷ Get Started Free
Patent: 55376
Estimated Expiration: ⤷ Get Started Free
Patent: 79854
Estimated Expiration: ⤷ Get Started Free
Patent: 28481
Estimated Expiration: ⤷ Get Started Free
Patent: 92346
Estimated Expiration: ⤷ Get Started Free
Patent: 54892
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1102425
Patent: ANTI-VIRAL COMPOUNDS.
Estimated Expiration: ⤷ Get Started Free
Patent: 1203502
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 1203503
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 1300112
Patent: SOLID COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 1302269
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 1705519
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 1903284
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1452916
Estimated Expiration: ⤷ Get Started Free
Patent: 1481395
Estimated Expiration: ⤷ Get Started Free
Patent: 1586215
Estimated Expiration: ⤷ Get Started Free
Patent: 1677481
Estimated Expiration: ⤷ Get Started Free
Patent: 1831154
Estimated Expiration: ⤷ Get Started Free
Patent: 1990936
Estimated Expiration: ⤷ Get Started Free
Patent: 2059386
Estimated Expiration: ⤷ Get Started Free
Patent: 120117620
Patent: ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Patent: 130053440
Patent: 고체 조성물 (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 140032338
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 140037974
Patent: ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION
Estimated Expiration: ⤷ Get Started Free
Patent: 140143152
Patent: ANTI-VIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 150008151
Patent: SOLID COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 180023014
Patent: 고체 조성물 (SOLID COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 180026775
Patent: 항바이러스 화합물 (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 14934
Estimated Expiration: ⤷ Get Started Free
Patent: 11440
Estimated Expiration: ⤷ Get Started Free
Patent: 26908
Estimated Expiration: ⤷ Get Started Free
Patent: 46767
Estimated Expiration: ⤷ Get Started Free
Patent: 60842
Estimated Expiration: ⤷ Get Started Free
Patent: 65536
Estimated Expiration: ⤷ Get Started Free
Patent: 24246
Estimated Expiration: ⤷ Get Started Free
Patent: 91625
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 02070
Estimated Expiration: ⤷ Get Started Free
Patent: 19874
Estimated Expiration: ⤷ Get Started Free
Patent: 69780
Estimated Expiration: ⤷ Get Started Free
Patent: 86159
Estimated Expiration: ⤷ Get Started Free
Patent: 87700
Estimated Expiration: ⤷ Get Started Free
Patent: 86660
Estimated Expiration: ⤷ Get Started Free
Patent: 21611
Estimated Expiration: ⤷ Get Started Free
Patent: 1102063
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1204713
Patent: Solid compositions
Estimated Expiration: ⤷ Get Started Free
Patent: 1238948
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1247648
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1334778
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1347759
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1412707
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1519891
Patent: Anti-viral compounds
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1815161
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 3052
Patent: ПРОТИВІРУСНІ СПОЛУКИ[ПРОТИВОВИРУСНЫЕ СОЕДИНЕНИЯ (ANTI-VIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 5434
Patent: ТВЕРДАЯ КОМПОЗИЦИЯ И СПОСОБ ЛЕЧЕНИЯ ГЕПАТИТА С;ТВЕРДА КОМПОЗИЦІЯ ТА СПОСІБ ЛІКУВАННЯ ВІРУСУ ГЕПАТИТУ С (SOLID COMPOSITION AND METHOD FOR TREATMENT OF HEPATITIS C)
Estimated Expiration: ⤷ Get Started Free
Patent: 8904
Patent: ПРОТИВІРУСНІ СПОЛУКИ
Estimated Expiration: ⤷ Get Started Free
Patent: 3048
Patent: ПРОТИВІРУСНІ СПОЛУКИ
Estimated Expiration: ⤷ Get Started Free
Patent: 8080
Patent: ПРОТИВІРУСНІ СПОЛУКИ
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 699
Patent: COMPUESTOS ANTIVIRALES EFICACES PARA INHIBIR LA REPLICACIÓN DEL VIRUS DE LA HEPATITIS C ("HCV"), PROCESOS, COMPOSICIONES, Y MÉTODOS RELACIONADOS
Estimated Expiration: ⤷ Get Started Free
Patent: 446
Patent: COMPOSICIONES SOLIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 667
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 983
Patent: COMPUESTOS ANTIVIRALES EFICACES PARA INHIBIR LA REPLICACIÓN DEL VIRUS DE LA HEPATITIS C ("HCV") Y C OMPOSICIONES RELACIONADAS
Estimated Expiration: ⤷ Get Started Free
Patent: 266
Patent: COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TECHNIVIE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Turkey | 201809771 | ⤷ Get Started Free | |
| European Patent Office | 2130534 | ⤷ Get Started Free | |
| Taiwan | 201519891 | Anti-viral compounds | ⤷ Get Started Free |
| European Patent Office | 2627651 | COMPOSÉS ANTIVIRAUX (ANTI-VIRAL COMPOUNDS) | ⤷ Get Started Free |
| European Patent Office | 2337781 | COMPOSÉS ANTIVIRAUX POUR LE TRAITEMENT D'INFECTIONS HCV (ANTI-VIRAL COMPOUNDS TO TREAT HCV INFECTION) | ⤷ Get Started Free |
| Luxembourg | 92667 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TECHNIVIE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2692346 | PA2017033 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: PIBRENTASVIRAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/17/1213 20170726 |
| 2368890 | SPC/GB15/015 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: OMBITASVIR AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU/1/14/982 20150119 |
| 2692346 | 2017/046 | Ireland | ⤷ Get Started Free | PRODUCT NAME: PIBRENTASVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; REGISTRATION NO/DATE: EU/1/17/1213 20170726 |
| 2692346 | SPC/GB17/056 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: PIBRENTASVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/17/1213 (NI) 20170728; UK PLGB 41042/0030 20170728; UK PLGB 41042/0043 20170728 |
| 2692346 | 1790050-7/1791051-4 | Sweden | ⤷ Get Started Free | PRODUCT NAME: PIBRENTASVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF. |
| 2340029 | SPC/GB15/014 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: PARITAPREVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT OR ESTER THEREOF; REGISTERED: UK EU/1/14/982 20150119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: TECHNIVIE
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
