TAMSULOSIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tamsulosin, and when can generic versions of Tamsulosin launch?
Tamsulosin is a drug marketed by Alkem Labs Ltd, Ascent Pharms Inc, Aurobindo Pharma Ltd, Chartwell Rx, Impax Labs, Macleods Pharms Ltd, Ph Health, Pharmobedient, Rising, Sandoz, Sciegen Pharms, Sun Pharm Inds Ltd, Synthon Bv, Teva Pharms, and Zydus Pharms Usa Inc. and is included in fifteen NDAs.
The generic ingredient in TAMSULOSIN is tamsulosin hydrochloride. There are thirty-three drug master file entries for this compound. Thirty-four suppliers are listed for this compound. Additional details are available on the tamsulosin hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tamsulosin
A generic version of TAMSULOSIN was approved as tamsulosin hydrochloride by IMPAX LABS on March 2nd, 2010.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TAMSULOSIN?
- What are the global sales for TAMSULOSIN?
- What is Average Wholesale Price for TAMSULOSIN?
Summary for TAMSULOSIN
| US Patents: | 0 |
| Applicants: | 15 |
| NDAs: | 15 |
| Drug Prices: | Drug price information for TAMSULOSIN |
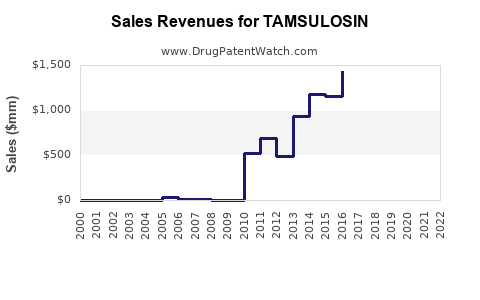
| Drug Sales Revenues: | Drug sales revenues for TAMSULOSIN |
| DailyMed Link: | TAMSULOSIN at DailyMed |
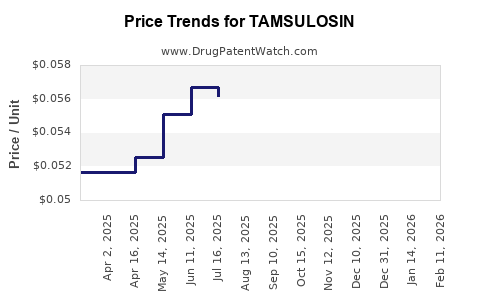
See drug prices for TAMSULOSIN
US Patents and Regulatory Information for TAMSULOSIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkem Labs Ltd | TAMSULOSIN HYDROCHLORIDE | tamsulosin hydrochloride | CAPSULE;ORAL | 207405-001 | Aug 11, 2017 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Ph Health | TAMSULOSIN HYDROCHLORIDE | tamsulosin hydrochloride | CAPSULE;ORAL | 202010-001 | Jan 4, 2013 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Chartwell Rx | TAMSULOSIN HYDROCHLORIDE | tamsulosin hydrochloride | CAPSULE;ORAL | 211885-001 | Oct 17, 2019 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
