TABRECTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tabrecta, and when can generic versions of Tabrecta launch?
Tabrecta is a drug marketed by Novartis Pharm and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eighty-two patent family members in forty-five countries.
The generic ingredient in TABRECTA is capmatinib hydrochloride. One supplier is listed for this compound. Additional details are available on the capmatinib hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Tabrecta
Tabrecta was eligible for patent challenges on May 6, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 22, 2035. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TABRECTA?
- What are the global sales for TABRECTA?
- What is Average Wholesale Price for TABRECTA?
Summary for TABRECTA
| International Patents: | 182 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 83 |
| Clinical Trials: | 1 |
| Drug Prices: | Drug price information for TABRECTA |
| What excipients (inactive ingredients) are in TABRECTA? | TABRECTA excipients list |
| DailyMed Link: | TABRECTA at DailyMed |
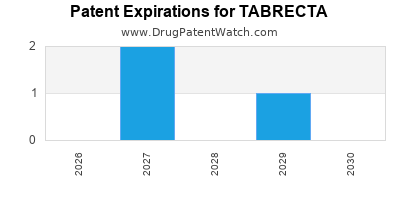

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TABRECTA
Generic Entry Date for TABRECTA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TABRECTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Timothy Burns | Phase 2 |
| Novartis | Phase 2 |
Pharmacology for TABRECTA
Paragraph IV (Patent) Challenges for TABRECTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TABRECTA | Tablets | capmatinib hydrochloride | 150 mg and 200 mg | 213591 | 1 | 2024-05-06 |
US Patents and Regulatory Information for TABRECTA
TABRECTA is protected by seven US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TABRECTA is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,596,178.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis Pharm | TABRECTA | capmatinib hydrochloride | TABLET;ORAL | 213591-001 | May 6, 2020 | RX | Yes | No | 12,084,449 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Novartis Pharm | TABRECTA | capmatinib hydrochloride | TABLET;ORAL | 213591-002 | May 6, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Novartis Pharm | TABRECTA | capmatinib hydrochloride | TABLET;ORAL | 213591-001 | May 6, 2020 | RX | Yes | No | 12,208,101 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for TABRECTA
When does loss-of-exclusivity occur for TABRECTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1286
Patent: FORMULACIÓN EN FORMA DE TABLETA DE UN INHIBIDOR DE C-MET
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 15293539
Patent: Tablet formulation of 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)benzamide
Estimated Expiration: ⤷ Get Started Free
Patent: 18207947
Patent: Tablet formulation of 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)Benzamide
Estimated Expiration: ⤷ Get Started Free
Patent: 20200912
Patent: Tablet formulation of 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)Benzamide
Estimated Expiration: ⤷ Get Started Free
Patent: 21202500
Patent: Tablet formulation of 2-fluoro-N-methyl-4-(7-(quinolin-6- ylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)Benzamide
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2017000953
Patent: formulação de comprimido de 2-flúor-n-metil-4-[7-(quinolin-6-il-metil)imidazo[1,2-b][1,2,4]triazin-2-il]benzamida
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 54840
Patent: FORMULATION DE COMPRIME DE 2-FLUORO-N-METHYL-4-[7-(QUINOLINE -6-YLMETHYL) IMIDAZO[1,2-B] [1,2,4]TRIAZINE -2-YL]BENZAMIDE (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 17000180
Patent: Formulacion en forma de tableta de 2-fluor-n-metil-4-[7-(quinolin-6-ilmetil)imidazo[1,2-b][1,2,4]triazin-2-il]benzamida.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 6714784
Patent: 2‑氟‑N‑甲基‑4‑[7‑(喹啉‑6‑基甲基)咪唑并[1,2‑b][1,2,4]三嗪‑2‑基]苯甲酰胺的片剂制剂 (Tablet formulation of 2-fluoro-n-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide)
Estimated Expiration: ⤷ Get Started Free
Patent: 5364061
Patent: C-MET抑制剂的片剂制剂 (Tablet formulations of C-MET inhibitors)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 17000586
Patent: Formulación en forma de tableta de 2-fluor-n-metil-4-[7-(quinolin-6-ylmetil)imidazo[1,2-b][1,2,4]triazin-2-il]benzamida
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 72209
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 17011672
Patent: FORMULACIÓN EN FORMA DE TABLETA DE 2-FLUOR-N-METIL-4-[7-(QUINOLIN-6-YLMETIL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-IL]BENZAMIDA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 9220
Patent: СОСТАВ ТАБЛЕТКИ 2-ФТОР-N-МЕТИЛ-4-[7-(ХИНОЛИН-6-ИЛМЕТИЛ)ИМИДАЗО[1,2-b][1,2,4]ТРИАЗИН-2-ИЛ]БЕНЗАМИДА (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1790259
Patent: СОСТАВ ТАБЛЕТОК 2-ФТОР-N-МЕТИЛ-4-[7-(ХИНОЛИН-6-ИЛМЕТИЛ)ИМИДАЗО[1,2-b][1,2,4]ТРИАЗИН-2-ИЛ]БЕНЗАМИДА
Estimated Expiration: ⤷ Get Started Free
Patent: 2191301
Patent: СОСТАВ ТАБЛЕТОК 2-ФТОР-N-МЕТИЛ-4-[7-(ХИНОЛИН-6-ИЛМЕТИЛ)ИМИДАЗО[1,2-b][1,2,4]ТРИАЗИН-2-ИЛ]БЕНЗАМИДА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 72209
Patent: FORMULATION DE COMPRIMÉ DE 2-FLUORO-N-MÉTHYL-4-[7-(QUINOLINE -6-YLMÉTHYL) IMIDAZO[1,2-B][1,2,4]TRIAZINE -2-YL]BENZAMIDE (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 48376
Patent: FORMULATION DE COMPRIMÉ D'UN INHIBITEUR DE C-MET (TABLET FORMULATION OF A C-MET INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1058
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1700007
Patent: FORMULACIÓN EN FORMA DE TABLETA DE 2-FLUOR-N-METIL-4-[7-(QUINOLIN-6-YLMETIL)IMIDAZO[1,2-B] [1,2,4]TRIAZIN-2-IL]BENZAMIDA
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 53346
Estimated Expiration: ⤷ Get Started Free
Patent: 200054
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 0166
Patent: פורמולצית טבליה של 2-פלואורו-n-מתיל-4-[7-(קווינולינ-6-ילמתיל)אימידזו[1, 2-b][1,2,4]טריאזינ-2-יל]בנזאמיד (Tablet formulation of 2-fluoro-n-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 70946
Estimated Expiration: ⤷ Get Started Free
Patent: 02587
Estimated Expiration: ⤷ Get Started Free
Patent: 17521469
Patent: 2−フルオロ−N−メチル−4−[7−(キノリン−6−イルメチル)イミダゾ[1,2−B][1,2,4]トリアジン−2−イル]ベンズアミドの錠剤
Estimated Expiration: ⤷ Get Started Free
Patent: 20114852
Patent: 2−フルオロ−N−メチル−4−[7−(キノリン−6−イルメチル)イミダゾ[1,2−B][1,2,4]トリアジン−2−イル]ベンズアミドの錠剤 (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL) IMIDAZO [1,2-B] [1,2,4] TRIAZIN-2-YL] BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 22046659
Patent: 2-フルオロ-N-メチル-4-[7-(キノリン-6-イルメチル)イミダゾ[1,2-B][1,2,4]トリアジン-2-イル]ベンズアミドの錠剤
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 18
Patent: صيغة قرص2-فلورو-Nميثيل -4 -[7-(كوينولين-6-يل ميثيل)اميدازو[2,1-B] [4,2,1] تريازين-2-يل] بنزاميد (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2,B] [1,2,4]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7276
Patent: TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9279
Patent: FORMULACION EN FORMA DE TABLETA DE 2-FLUOR-N-METIL-4-[7-(QUINOLIN-6-YLMETIL) IMIDAZO[1,2,B] [1,2,4]TRIAZIN-2-IL]BENZAMIDA. (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 17001177
Patent: FORMULACION EN FORMA DE TABLETA DE 2-FLUOR-N-METIL-4-[7-(QUINOLIN- 6-YLMETIL) IMIDAZO[1,2,B] [1,2,4]TRIAZIN-2-IL]BENZAMIDA. (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL )IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21000595
Patent: FORMULACION EN FORMA DE TABLETA DE 2-FLUOR-N-METIL-4-[7-(QUINOLIN- 6-YLMETIL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-IL]BENZAMIDA. (TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL )IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE.)
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1208
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8089
Patent: Tablet formulation of 2-fluoro-n-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 22058
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 170523
Patent: FORMULACION EN FORMA DE TABLETA DE 2-FLUOR-N-METIL-4-[7-(QUINOLIN-6-YLMETIL)IMIDAZO[1,2- B][1,2,4]TRIAZIN-2-IL]BENZAMIDA
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 017500121
Patent: TABLET FORMULATION OF A C-MET INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 72209
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 72209
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201900648S
Patent: TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE
Estimated Expiration: ⤷ Get Started Free
Patent: 201700147S
Patent: TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YLMETHYL)IMIDAZO[1,2-B][1,2,4]TRIAZIN-2-YL]BENZAMIDE
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 72209
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2581121
Estimated Expiration: ⤷ Get Started Free
Patent: 170039211
Patent: 2-플루오로-N-메틸-4-[7-이미다조[1,2-B][1,2,4]트리아진-2-일]벤즈아미드의 정제 제제 (2--N--4-[7--6-[12-B][124]-2-] TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-QUINOLIN-6-YLMETHYLIMIDAZO[12-B][124]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 230136693
Patent: 2-플루오로-N-메틸-4-[7-이미다조[1,2-B][1,2,4]트리아진-2-일]벤즈아미드의 정제 제제 (2--N--4-[7--6-[12-B][124]-2-] TABLET FORMULATION OF 2-FLUORO-N-METHYL-4-[7-QUINOLIN-6-YLMETHYLIMIDAZO[12-B][124]TRIAZIN-2-YL]BENZAMIDE)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 57523
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 24993
Estimated Expiration: ⤷ Get Started Free
Patent: 1613595
Patent: Tablet formulation of a C-MET inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 2200148
Patent: Tablet formulation of a C-MET inhibitor
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TABRECTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | 200835481 | ⤷ Get Started Free | |
| Croatia | P20181622 | ⤷ Get Started Free | |
| Israel | 276928 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TABRECTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2099447 | C02099447/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: CAPMATINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67648 26.04.2021 |
| 3172209 | C202230060 | Spain | ⤷ Get Started Free | PRODUCT NAME: CAPMATINIB O UNA SAL FARMACEUTICAMENTE ACEPTABLE DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/22/1650; DATE OF AUTHORISATION: 20220620; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/22/1650; DATE OF FIRST AUTHORISATION IN EEA: 20220620 |
| 2099447 | PA2022527,C2099447 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: KAPMATINIBAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/22/1650 20220620 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TABRECTA (Erdafitinib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
