SYMLIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Symlin, and what generic alternatives are available?
Symlin is a drug marketed by Astrazeneca Ab and is included in one NDA.
The generic ingredient in SYMLIN is pramlintide acetate. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the pramlintide acetate profile page.
Summary for SYMLIN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 11 |
| Clinical Trials: | 20 |
| Patent Applications: | 2,786 |
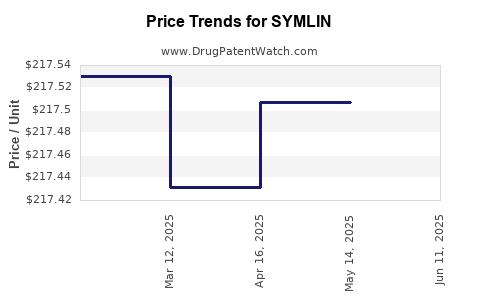
| Drug Prices: | Drug price information for SYMLIN |
| DailyMed Link: | SYMLIN at DailyMed |
Recent Clinical Trials for SYMLIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Institute on Aging (NIA) | Early Phase 1 |
| Boston University | Early Phase 1 |
| H. Lee Moffitt Cancer Center and Research Institute | Early Phase 1 |
Pharmacology for SYMLIN
| Drug Class | Amylin Analog |
| Mechanism of Action | Amylin Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for SYMLIN
US Patents and Regulatory Information for SYMLIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-002 | Sep 25, 2007 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-003 | Sep 25, 2007 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-001 | Mar 16, 2005 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SYMLIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-003 | Sep 25, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-002 | Sep 25, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-003 | Sep 25, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-002 | Sep 25, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for SYMLIN
See the table below for patents covering SYMLIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2077265 | ⤷ Try a Trial | |
| Japan | S6463594 | PEPTIDES | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 9220366 | ⤷ Try a Trial | |
| Japan | 2003238594 | NEW AMYLIN AGONIST PEPTIDE AND USE THEREFOR | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


