STEGLATRO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Steglatro, and when can generic versions of Steglatro launch?
Steglatro is a drug marketed by Msd Sub Merck and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has sixty-one patent family members in forty-nine countries.
The generic ingredient in STEGLATRO is ertugliflozin. One supplier is listed for this compound. Additional details are available on the ertugliflozin profile page.
DrugPatentWatch® Generic Entry Outlook for Steglatro
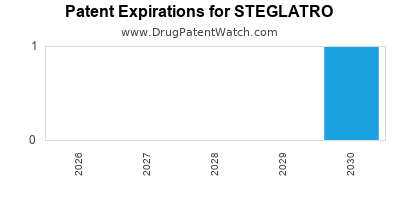
Steglatro was eligible for patent challenges on December 19, 2021.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for STEGLATRO?
- What are the global sales for STEGLATRO?
- What is Average Wholesale Price for STEGLATRO?
Summary for STEGLATRO
| International Patents: | 61 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 56 |
| Clinical Trials: | 4 |
| Patent Applications: | 1,067 |
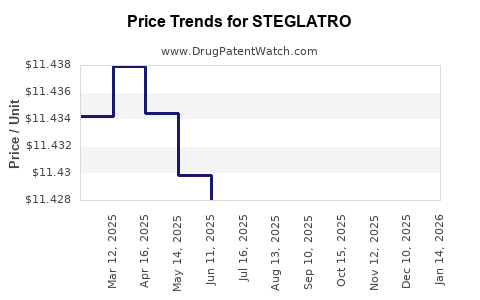
| Drug Prices: | Drug price information for STEGLATRO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for STEGLATRO |
| What excipients (inactive ingredients) are in STEGLATRO? | STEGLATRO excipients list |
| DailyMed Link: | STEGLATRO at DailyMed |
Recent Clinical Trials for STEGLATRO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Klinik Ottakring | Phase 3 |
| Wilhelminenspital Vienna | Phase 3 |
| Klinikum Wiener Neustadt | Phase 3 |
Paragraph IV (Patent) Challenges for STEGLATRO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| STEGLATRO | Tablets | ertugliflozin | 5 mg and 15 mg | 209803 | 3 | 2021-12-20 |
US Patents and Regulatory Information for STEGLATRO
STEGLATRO is protected by one US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msd Sub Merck | STEGLATRO | ertugliflozin | TABLET;ORAL | 209803-001 | Dec 19, 2017 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Msd Sub Merck | STEGLATRO | ertugliflozin | TABLET;ORAL | 209803-002 | Dec 19, 2017 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for STEGLATRO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme B.V. | Steglatro | ertugliflozin | EMEA/H/C/004315Steglatro is indicated in adults aged 18 years and older with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control:as monotherapy in patients for whom the use of metformin is considered inappropriate due to intolerance or contraindications.in addition to other medicinal products for the treatment of diabetes. | Authorised | no | no | no | 2018-03-21 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for STEGLATRO
When does loss-of-exclusivity occur for STEGLATRO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 28
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 3138
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09286380
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 40040
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0918841
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 33795
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11000394
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2149717
Estimated Expiration: ⤷ Get Started Free
Patent: 3497199
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 41636
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 110077
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120104
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 003
Estimated Expiration: ⤷ Get Started Free
Patent: 110041
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 12497
Estimated Expiration: ⤷ Get Started Free
Patent: 18024
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 34687
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 011000058
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 11010854
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 11003842
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8492
Estimated Expiration: ⤷ Get Started Free
Patent: 1100266
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 34687
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1036
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0135803
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 09001652
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 56616
Estimated Expiration: ⤷ Get Started Free
Patent: 93606
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 800031
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1226
Estimated Expiration: ⤷ Get Started Free
Patent: 6804
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 25322
Estimated Expiration: ⤷ Get Started Free
Patent: 12500842
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 334687
Estimated Expiration: ⤷ Get Started Free
Patent: 2018510
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 5418
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 11002166
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 285
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 590
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 0943
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1027
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1100043
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 18019
Estimated Expiration: ⤷ Get Started Free
Panama
Patent: 40801
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 110288
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 34687
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 34687
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 236
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 34687
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1101341
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1338540
Estimated Expiration: ⤷ Get Started Free
Patent: 1446454
Estimated Expiration: ⤷ Get Started Free
Patent: 110045093
Estimated Expiration: ⤷ Get Started Free
Patent: 130116078
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 80408
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 87598
Estimated Expiration: ⤷ Get Started Free
Patent: 1014863
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 11000066
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 3626
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 073
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering STEGLATRO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Malaysia | 155418 | ⤷ Get Started Free | |
| Israel | 211226 | ⤷ Get Started Free | |
| El Salvador | 2011003842 | ⤷ Get Started Free | |
| China | 103497199 | ⤷ Get Started Free | |
| Panama | 8840801 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for STEGLATRO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2334687 | CA 2018 00025 | Denmark | ⤷ Get Started Free | PRODUCT NAME: ERTUGLIFLOZIN, EVENTUELT SOM EN KRYSTALFORM, SAERLIGT SOM ET CO-KRYSTAL MED L-PYROGLUTAMINSYRE OG SPECIFIKT SOM ERTUGLIFLOZIN-L-PYROGLUTAMINSYRE; NAT. REG. NO/DATE: EU/1/18/1267/001-012 20180323; FIRST REG. NO/DATE: EU EU/1/18/1267/001/012 20180323 |
| 2334687 | C02334687/01 | Switzerland | ⤷ Get Started Free | FORMER OWNER: PFIZER INC., US |
| 2334687 | 300943 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ERTUGLIFLOZINE, DESGEWENST IN KRISTALVORM, MET NAME ALS CO-KRISTAL MET L-PYROGLUTAMINEZUUR, MET NAME ERTUGLIFLOZIN L-PYROGLUTAMINE ZUUR; REGISTRATION NO/DATE: EU/1/18/1267 20180323 |
| 2334687 | 716 | Finland | ⤷ Get Started Free | |
| 2334687 | 2018/028 | Ireland | ⤷ Get Started Free | PRODUCT NAME: ERTUGLIFLOZIN, OPTIONALLY AS A CRYSTAL FORM, PARTICULARLY AS A CO-CRYSTAL WITH L-PYROGLUTAMIC ACID, AND SPECIFICALLY AS ERTUGLIFLOZIN L-PYROGLUTAMIC ACID; REGISTRATION NO/DATE: EU/1/18/1267/001 EU/1/18/1267/012 20180321 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for STEGLATRO (Ertugliflozin)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
