SPRAVATO Drug Patent Profile
✉ Email this page to a colleague
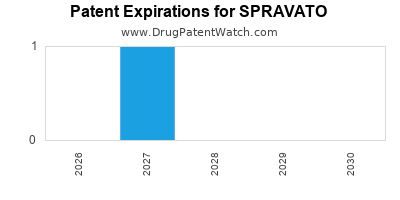
When do Spravato patents expire, and when can generic versions of Spravato launch?
Spravato is a drug marketed by Janssen Pharms and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has sixty patent family members in twenty-four countries.
The generic ingredient in SPRAVATO is esketamine hydrochloride. One supplier is listed for this compound. Additional details are available on the esketamine hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Spravato
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for SPRAVATO?
- What are the global sales for SPRAVATO?
- What is Average Wholesale Price for SPRAVATO?
Summary for SPRAVATO
| International Patents: | 60 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 18 |
| Clinical Trials: | 4 |
| Patent Applications: | 265 |
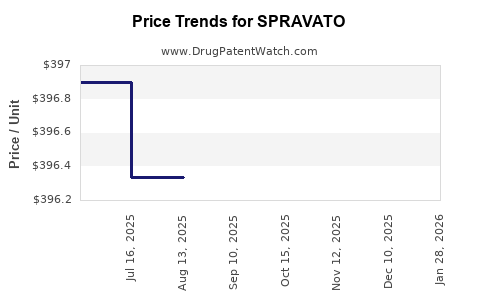
| Drug Prices: | Drug price information for SPRAVATO |
| What excipients (inactive ingredients) are in SPRAVATO? | SPRAVATO excipients list |
| DailyMed Link: | SPRAVATO at DailyMed |
Recent Clinical Trials for SPRAVATO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Royal North Shore Hospital | Phase 4 |
| Janssen-Cilag Pty Ltd | Phase 4 |
| VA Office of Research and Development | Phase 4 |
Paragraph IV (Patent) Challenges for SPRAVATO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SPRAVATO | Nasal Spray | esketamine hydrochloride | 28 mg | 211243 | 3 | 2023-03-06 |
US Patents and Regulatory Information for SPRAVATO
SPRAVATO is protected by fifteen US patents and one FDA Regulatory Exclusivity.
Patents protecting SPRAVATO
Methods for the treatment of depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DEPRESSIVE SYMPTOMS IN ADULTS WITH MDD WITH ACUTE SUICIDAL IDEATION OR BEHAVIOR BY NASALLY ADMINISTERING 56MG OR 84 MG OF ESKETAMINE 2X WEEKLY FOR 4 WEEKS IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Methods for the treatment of depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TRD IN ADULTS BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE 2X WEEKLY FOR 4 WEEKS DURING THE INDUCTION PHASE IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Methods for the treatment of depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TRD IN ADULTS BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE 2X WEEKLY FOR 4 WEEKS DURING THE INDUCTION PHASE FOLLOWED BY A MAINTENANCE PHASE OF 56MG OR 84 MG WEEKLY OR 1X EVERY TWO WEEKS IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DEPRESSION IN ADULTS WITH MOD AND ACUTE SUICIDAL IDEATION OR BEHAVIOR IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE IN A MAINTENANCE PHASE WEEKLY OR LX EVERY 2 WEEKS AFTER INDUCTION PHASE
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TRD IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE IN A MAINTENANCE PHASE WEEKLY OR 1X EVERY TWO WEEKS TO ADULTS WHO HAVE BEEN ADMINISTERED ESKETAMINE IN A INDUCTION PHASE FOR ABOUT 4 WEEKS
Methods for the treatment of depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TRD IN ADULTS BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE 2X WEEKLY FOR 4 WEEKS DURING THE INDUCTION PHASE IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Methods for the treatment of depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TRD IN ADULTS BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE 2X WEEKLY FOR 4 WEEKS DURING THE INDUCTION PHASE FOLLOWED BY A MAINTENANCE PHASE OF 56MG OR 84 MG WEEKLY OR 1X EVERY TWO WEEKS IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Methods for the treatment of depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DEPRESSIVE SYMPTOMS IN ADULTS WITH MDD WITH ACUTE SUICIDAL IDEATION OR BEHAVIOR BY NASALLY ADMINISTERING 56MG OR 84 MG OF ESKETAMINE 2X WEEKLY FOR 4 WEEKS IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DEPRESSIVE SYMPTOMS IN ADULTS WITH MDD WITH ACUTE SUICIDAL IDEATION OR BEHAVIOR BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE AS A PHARMACEUTICAL COMPOSITION TWICE PER WEEK IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TREATMENT-RESISTANT DEPRESSION IN ADULTS BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE TWICE PER WEEK AS A PHARMACEUTICAL COMPOSITION IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TREATMENT-RESISTANT DEPRESSION IN ADULTS BY NASALLY ADMINISTERING 56MG OR 84MG OF ESKETAMINE AS A PHARMACEUTICAL COMPOSITION IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DEPRESSION IN MDD WITH ACUTE SUICIDAL IDEATION/BEHAVIOR WITH NASALLY ADMINISTERED ESKETAMINE WITH OAD IN A PATIENT WHO HAS MISSED A DOSE IN THE MAINTENANCE PHASE AND HAD WORSENING DEPRESSION SYMPTOMS BY RETURN TO HIGHER DOSING SCHEDULE
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TRD WITH NASALLY ADMINISTERED ESKETAMINE IN CONJUNCTION WITH AN OAD IN A PATIENT WHO HAS MISSED A DOSE DURING THE MAINTENANCE PHASE AND HAD WORSENING OF DEPRESSION SYMPTOM BY RETURNING TO HIGHER DOSING SCHEDULE
Intranasal administration of ketamine to treat depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TREATMENT-RESISTANT DEPRESSION IN ADULT IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
Intranasal administration of ketamine to treat depression
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF TREATMENT-RESISTANT DEPRESSION IN ADULT IN CONJUNCTION WITH AN ORAL ANTIDEPRESSANT
FDA Regulatory Exclusivity protecting SPRAVATO
NEW CHEMICAL ENTITY (AN ENANTIOMER OF PREVIOUSLY APPROVED RACEMIC MIXTURE. SEE SECTION 505(U) OF THE FEDERAL FOOD AND DRUG COSMETIC ACT).
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Pharms | SPRAVATO | esketamine hydrochloride | SPRAY;NASAL | 211243-001 | Mar 5, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Janssen Pharms | SPRAVATO | esketamine hydrochloride | SPRAY;NASAL | 211243-001 | Mar 5, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Janssen Pharms | SPRAVATO | esketamine hydrochloride | SPRAY;NASAL | 211243-001 | Mar 5, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Janssen Pharms | SPRAVATO | esketamine hydrochloride | SPRAY;NASAL | 211243-001 | Mar 5, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for SPRAVATO
See the table below for patents covering SPRAVATO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Philippines | 12015502013 | PHARMACEUTICAL COMPOSITION OF S-KETAMINE HYDROCHLORIDE | ⤷ Sign Up |
| Morocco | 40620 | RÉGIMES POSOLOGIQUES SPÉCIFIQUES AU GÉNOTYPE VAL66MET (SNP RS6265) ET MÉTHODES POUR LE TRAITEMENT DE LA DÉPRESSION (VAL66MET (SNP rs6265) GENOTYPE SPECIFIC DOSING REGIMENS AND METHODS FOR THE TREATMENT OF DEPRESSION) | ⤷ Sign Up |
| Nicaragua | 201800038 | ⤷ Sign Up | |
| South Korea | 20150132381 | S-케타민 하이드로클로라이드의 약제학적 조성물 (PHARMACEUTICAL COMPOSITION OF S-KETAMINE HYDROCHLORIDE) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


