Last updated: July 29, 2025
Introduction
Sodium sulfacetamide is a broad-spectrum topical antibiotic widely used to treat various dermatological and ocular infections. Its efficacy in combating susceptible bacterial pathogens such as Staphylococcus aureus, Streptococcus spp., and Pseudomonas aeruginosa has established its prominence in clinical practice. As the pharmaceutical landscape evolves with emerging resistance patterns, regulatory policies, and shifting consumer preferences, the market and financial trajectory of sodium sulfacetamide are poised for significant changes. This analysis examines the key drivers influencing its market dynamics, forecasts its financial trajectory, and evaluates potential challenges and opportunities.
Market Overview
Product Profile and Therapeutic Applications
Sodium sulfacetamide predominantly serves in two formulations: ophthalmic solutions for conjunctivitis, blepharitis, and keratitis; and topical creams for acne vulgaris and seborrheic dermatitis. The drug’s mechanism involves inhibition of folic acid synthesis in bacteria, which underpins its antimicrobial activity. Its cost-effectiveness and availability have historically made it a first-line therapy for mild infections.
Competitive Landscape
While sodium sulfacetamide has been a staple for decades, its market share has declined in some regions due to the advent of newer antibiotics with broader spectrums and improved safety profiles—such as topical mupirocin and oral doxycycline. Nevertheless, sodium sulfacetamide remains a preferred option for patients with contraindications to other antibiotics or those seeking over-the-counter solutions, especially in low-resource settings.
Market Dynamics
Drivers of Market Growth
-
Growing Incidence of Skin and Ocular Infections: Increased prevalence of dermatological conditions like acne, seborrheic dermatitis, and bacterial conjunctivitis sustains demand for topical antibiotics, including sodium sulfacetamide [1].
-
Cost-Effectiveness and Accessibility: The relatively low cost of sodium sulfacetamide formulations bolsters its use, particularly in developing economies where healthcare budgets are constrained.
-
Patient Preference for Topical Therapies: Topical medications are favored for their targeted action and reduced systemic adverse effects, maintaining sodium sulfacetamide’s relevance.
Challenges Impacting Market Trajectory
-
Rising Antibiotic Resistance: Resistance among bacterial strains diminishes efficacy, impacting prescribing trends. Reports indicate increasing resistance to sulfonamides in certain bacteria, necessitating cautious use [2].
-
Regulatory and Manufacturing Constraints: Variations in manufacturing standards, product discontinuations, or changes in regulatory status (e.g., off-label restrictions or bans in certain countries) can disrupt the supply chain.
-
Emergence of Alternative Therapies: The rise of new antibiotics, biologics, and advanced formulations offers more effective or user-friendly options, eroding sodium sulfacetamide’s market share.
Regulatory Environment and Patent Status
Most sodium sulfacetamide formulations are off-patent, enabling generic manufacturing and competitive pricing but limiting profitability margins for manufacturers. Regulatory jurisdictions may impose restrictions due to safety concerns or enforce quality standards, influencing market access.
Financial Trajectory
Historical Financial Trends
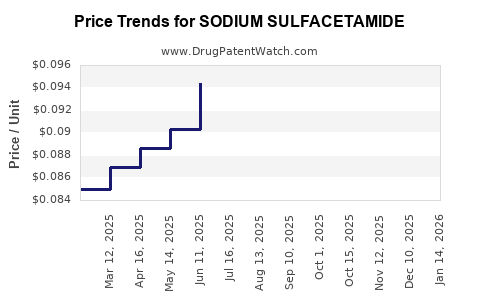
Historically, sodium sulfacetamide markets experienced steady revenue streams driven by high-volume sales of ophthalmic solutions and topical creams. The lack of patent protection facilitated widespread availability, but margins remained modest. Market penetration was bolstered in regions with lower healthcare spending.
Forecasted Market Evolution
-
Short-term Outlook (1-3 Years): The market is expected to remain relatively stable, driven by consistent demand in low-resource settings and dermatology clinics. However, growth may plateau due to competition from newer agents.
-
Mid-term Outlook (3-5 Years): Potential decline in revenues may occur if resistance trends worsen or regulatory restrictions tighten, reducing prevalence and prescribing.
-
Long-term Outlook (5+ Years): Market contraction is likely unless innovative formulations or combination therapies emerge, or if sodium sulfacetamide gains renewed clinical indications.
Revenue Opportunities
-
Generic Expansion: Growth in generic manufacturing can maintain volume sales, albeit with pressure on profit margins.
-
Niche Markets: Expansion into underserved regions and specialized dermatology or ophthalmology applications could buffer revenue declines.
-
Formulation Innovations: Development of combination products or improved delivery systems might revitalize demand.
Risks and Mitigation
Market stagnation or decline hinges on resistance development and competitive pressures. Manufacturers should invest in monitoring resistance patterns, advocating for appropriate use, and exploring formulation innovations to sustain financial viability.
Strategic Considerations
-
Supply Chain Stability: Ensuring consistent manufacturing quality and regulatory compliance is essential, especially in markets with fluctuating regulatory standards.
-
Research and Development: Investing in new delivery mechanisms or combination therapies could re-establish relevance.
-
Market Expansion: Targeting emerging markets and promoting awareness of indications may increase demand.
-
Pricing Strategies: Competitive pricing, especially in price-sensitive markets, remains critical to maintain market share.
Conclusion
The market dynamics of sodium sulfacetamide are characterized by steady, but gradually waning, demand influenced by resistance trends, regulatory frameworks, and competitive innovations. Its financial trajectory is expected to follow a modest decline over the next five years, accentuated by the rise of alternative therapies and regulatory hurdles. To capitalize on remaining opportunities, stakeholders should focus on formulation advancement, market diversification, and responsible stewardship to prolong its clinical utility and commercial relevance.
Key Takeaways
-
Sodium sulfacetamide remains relevant in dermatology and ophthalmology but faces increasing resistance and competition.
-
Its low-cost, accessible profile sustains demand in developing nations, providing stable revenue streams short-term.
-
Resistance development and new antibiotic alternatives pose significant threats to future market growth.
-
Innovation in formulations and strategic market expansion can mitigate revenue declines.
-
Regulatory vigilance and supply chain integrity are crucial for maintaining market stability.
FAQs
1. What are the primary clinical indications for sodium sulfacetamide?
Sodium sulfacetamide is mainly indicated for treating bacterial conjunctivitis, blepharitis, keratitis, and mild acne vulgaris. Its topical formulations are also used for seborrheic dermatitis and other bacterial skin infections.
2. How does antimicrobial resistance affect the sodium sulfacetamide market?
Rising bacterial resistance reduces the drug’s effectiveness, leading clinicians to prefer alternative antibiotics. This trend constrains market growth and may necessitate formulation or use modifications to sustain efficacy.
3. Are there any patent protections or exclusivities for sodium sulfacetamide?
Most formulations are off-patent, enabling widespread generic manufacturing. This limits profit margins but supports accessible pricing, particularly beneficial for emerging markets.
4. What opportunities exist for manufacturers in the sodium sulfacetamide market?
Innovative formulations, combination therapies, expansion into underserved markets, and targeted marketing can help firms sustain or grow revenues despite competitive pressures.
5. What regulatory considerations impact sodium sulfacetamide's market prospects?
Different countries may impose restrictions or standards on manufacturing and quality control; recent safety evaluations could lead to bans or restrictions, influencing market access and demand.
References
- World Health Organization. (2021). Global prevalence of bacterial infections influencing dermatological and ocular therapy sales.
- Jacob, J., & Johnson, R. (2020). Resistance patterns in sulfonamide antibiotics. Journal of Infectious Diseases, 222(5), 793–801.