SKLICE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Sklice, and what generic alternatives are available?
Sklice is a drug marketed by Arbor Pharms Llc and is included in one NDA.
The generic ingredient in SKLICE is ivermectin. There are five drug master file entries for this compound. Nineteen suppliers are listed for this compound. Additional details are available on the ivermectin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Sklice
A generic version of SKLICE was approved as ivermectin by EDENBRIDGE PHARMS on October 24th, 2014.
Summary for SKLICE
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 103 |
| Clinical Trials: | 5 |
| Formulation / Manufacturing: | see details |
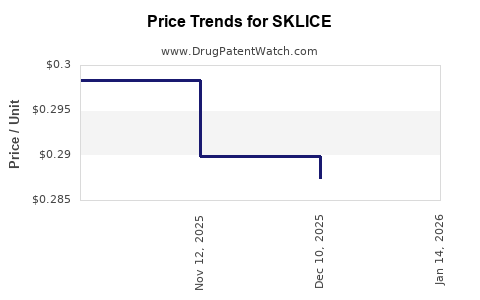
| Drug Prices: | Drug price information for SKLICE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SKLICE |
| What excipients (inactive ingredients) are in SKLICE? | SKLICE excipients list |
| DailyMed Link: | SKLICE at DailyMed |
Recent Clinical Trials for SKLICE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 2 |
| City of Hope Medical Center | Phase 2 |
| Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Phase 2 |
Pharmacology for SKLICE
| Drug Class | Antiparasitic Pediculicide |
Anatomical Therapeutic Chemical (ATC) Classes for SKLICE
Paragraph IV (Patent) Challenges for SKLICE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SKLICE | Lotion | ivermectin | 0.50% | 202736 | 1 | 2017-09-01 |
US Patents and Regulatory Information for SKLICE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arbor Pharms Llc | SKLICE | ivermectin | LOTION;TOPICAL | 202736-001 | Feb 7, 2012 | OTC | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SKLICE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Arbor Pharms Llc | SKLICE | ivermectin | LOTION;TOPICAL | 202736-001 | Feb 7, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for SKLICE
See the table below for patents covering SKLICE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| New Zealand | 700972 | Preservative system for emulsion-based therapeutic topical formulations | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2008137699 | ⤷ Sign Up | |
| European Patent Office | 2091325 | Formulation shampooing-après-shampooing comprenant un avermectine pour l'élimination et la prophylaxie contre les souches de poux sensibles et résistantes aux traitements (Shampoo-conditioner formulations comprising an avermectin for elimination of, and prophylaxis against, susceptible and treatment-resistant strains of lice) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SKLICE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1620113 | C01620113/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: IVERMECTINUM; REGISTRATION NO/DATE: AUTORISATION SWISSMEDIC 65561 20.12.2016 |
| 1620113 | 122015000079 | Germany | ⤷ Sign Up | PRODUCT NAME: IVERMECTIN ZU SEINER VERWENDUNG FUER DIE BEHANDLUNG VON ROSAZEA; NAT. REGISTRATION NO/DATE: 92429.00.00 20150429; FIRST REGISTRATION: MALTA MA 117/01101 20150402 |
| 1620113 | 300756 | Netherlands | ⤷ Sign Up | PRODUCT NAME: IVERMECTINE VOOR TOEPASSING IN DE BEHANDELING VAN ROSACEA; NATIONAL REGISTRATION NO/DATE: RVG 115310 20150504; FIRST REGISTRATION: MT MA117/01101 20150402 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


