QSYMIA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Qsymia, and when can generic versions of Qsymia launch?
Qsymia is a drug marketed by Vivus Llc and is included in one NDA. There are six patents protecting this drug and one Paragraph IV challenge.
This drug has forty patent family members in seventeen countries.
The generic ingredient in QSYMIA is phentermine hydrochloride; topiramate. There are seventeen drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the phentermine hydrochloride; topiramate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Qsymia
A generic version of QSYMIA was approved as phentermine hydrochloride; topiramate by ACTAVIS LABS FL INC on June 25th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for QSYMIA?
- What are the global sales for QSYMIA?
- What is Average Wholesale Price for QSYMIA?
Summary for QSYMIA
| International Patents: | 40 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 4 |
| Clinical Trials: | 20 |
| Drug Prices: | Drug price information for QSYMIA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for QSYMIA |
| What excipients (inactive ingredients) are in QSYMIA? | QSYMIA excipients list |
| DailyMed Link: | QSYMIA at DailyMed |

Recent Clinical Trials for QSYMIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Minnesota | PHASE3 |
| University of Toronto | PHASE4 |
| Mayo Clinic | PHASE4 |
Pharmacology for QSYMIA
| Drug Class | Sympathomimetic Amine Anorectic |
| Mechanism of Action | Cytochrome P450 2C19 Inhibitors Cytochrome P450 3A4 Inducers |
| Physiological Effect | Appetite Suppression Decreased Central Nervous System Disorganized Electrical Activity Increased Sympathetic Activity |
Paragraph IV (Patent) Challenges for QSYMIA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| QSYMIA | Extended-release Capsules | phentermine hydrochloride; topiramate | 3.75 mg/23 mg 7.5 mg/46 mg 11.25 mg/69 mg 15 mg/92 mg | 022580 | 1 | 2013-07-18 |
US Patents and Regulatory Information for QSYMIA
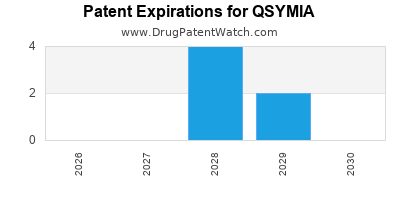
QSYMIA is protected by six US patents and two FDA Regulatory Exclusivities.
Expired US Patents for QSYMIA
International Patents for QSYMIA
When does loss-of-exclusivity occur for QSYMIA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09257572
Estimated Expiration: ⤷ Get Started Free
Patent: 09257573
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0914985
Estimated Expiration: ⤷ Get Started Free
Patent: 0914991
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 27313
Estimated Expiration: ⤷ Get Started Free
Patent: 27319
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 10001365
Estimated Expiration: ⤷ Get Started Free
Patent: 10001366
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2112126
Estimated Expiration: ⤷ Get Started Free
Patent: 2112127
Estimated Expiration: ⤷ Get Started Free
Patent: 4825477
Estimated Expiration: ⤷ Get Started Free
Patent: 5534921
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18103
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 17997
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 00002
Estimated Expiration: ⤷ Get Started Free
Patent: 17997
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 13489
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9874
Patent: תכשיר טופירמאט/פנטרמין בעל מינון נמוך ושיטות לשימוש בו (Low dose topiramate/phentermine composition and methods of use thereof)
Estimated Expiration: ⤷ Get Started Free
Patent: 9875
Patent: Topiramate ו- phentermine לצורך ירידה במשקל במשטר מינון עולה (Topiramate and phentermine for effecting weight loss in an escalating dosing regimen)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 52595
Estimated Expiration: ⤷ Get Started Free
Patent: 77053
Estimated Expiration: ⤷ Get Started Free
Patent: 14750
Estimated Expiration: ⤷ Get Started Free
Patent: 11522896
Estimated Expiration: ⤷ Get Started Free
Patent: 11522897
Estimated Expiration: ⤷ Get Started Free
Patent: 15166380
Patent: 体重減少の達成および肥満症の治療のための漸増用量投与計画(ESCALATINGDOSINGREGIMEN) (ESCALATING DOSING REGIMEN (ESCALATINGDOSINGREGIMEN) FOR ACHIEVING WEIGHT REDUCTION AND TREATING OBESITY)
Estimated Expiration: ⤷ Get Started Free
Patent: 16006085
Patent: 低用量トピラメート/フェンテルミン組成物およびその使用方法 (LOW DOSE TOPIRAMATE/PHENTERMINE COMPOSITION AND METHODS OF USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 17078083
Patent: 低用量トピラメート/フェンテルミン組成物およびその使用方法 (LOW DOSE TOPIRAMATE/PHENTERMINE COMPOSITION AND METHODS OF USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 17105788
Patent: 体重減少の達成および肥満症の治療のための漸増用量投与計画(ESCALATING DOSING REGIMEN) (ESCALATING DOSING REGIMEN FOR EFFECTING WEIGHT LOSS AND TREATING OBESITY)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 2684
Patent: REGIMEN DE DOSIFICACION ESCALANTE PARA EFECTUAR LA PERDIDA DE PESO Y EL TRATAMIENTO DE OBESIDAD. (ESCALATING DOSING REGIMEN FOR EFFECTING WEIGHT LOSS AND TREATING OBESITY.)
Estimated Expiration: ⤷ Get Started Free
Patent: 10013503
Patent: COMPOSICION DE TOPIRAMATO/FENTERMINA DE BAJA DOSIS Y METODOS DE USO DE LA MISMA. (LOW DOSE TOPIRAMATE/PHENTERMINE COMPOSTION AND METHODS OF USE THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 10013505
Patent: REGIMEN DE DOSIFICACION ESCALANTE PARA EFECTUAR LA PERDIDA DE PESO Y EL TRATAMIENTO DE OBESIDAD. (ESCALATING DOSING REGIMEN FOR EFFECTING WEIGHT LOSS AND TREATING OBESITY.)
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 17997
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1008839
Patent: LOW DOSE TOPIRAMATE/PHENTERMINE COMPOSITION AND METHODS OF USE THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 1008840
Patent: ESCALATING DOSING REGIMEN FOR EFFECTING WEIGHT LOSS AND TREATING OBESITY
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 110042280
Patent: LOW DOSE TOPIRAMATE/PHENTERMINE COMPOSITION AND METHODS OF USE THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 110044847
Estimated Expiration: ⤷ Get Started Free
Patent: 140121491
Patent: ESCALATING DOSING REGIMEN FOR EFFECTING WEIGHT LOSS AND TREATING OBESITY
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 06041
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering QSYMIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Israel | 127715 | ANTICONVULSANT SULFAMATE DERIVATIVES USEFUL IN TREATING OBESITY | ⤷ Get Started Free |
| World Intellectual Property Organization (WIPO) | 2008153632 | ⤷ Get Started Free | |
| China | 102112126 | ⤷ Get Started Free | |
| European Patent Office | 2305227 | Thérapie combinée pour le traitement du diabète associé à l'obésité (Combination therapy for the treatment of diabetes associated with obesity) | ⤷ Get Started Free |
| Hong Kong | 1213489 | ⤷ Get Started Free | |
| Australia | 732923 | ⤷ Get Started Free | |
| Spain | 2291215 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for QSYMIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2317997 | CR 2021 00049 | Denmark | ⤷ Get Started Free | PRODUCT NAME: PHENTERMIN OG TOPIRAMAT; NAT. REG. NO/DATE: 63166, 63167, 63168, 63169 20210705; FIRST REG. NO/DATE: IS IS/1/21/018/01-04 20210212 |
| 2317997 | 833 | Finland | ⤷ Get Started Free | |
| 2317997 | CA 2021 00049 | Denmark | ⤷ Get Started Free | PRODUCT NAME: PHENTERMIN OG TOPIRAMAT; NAT. REG. NO/DATE: 63166, 63167, 63168, 63169 20210705; FIRST REG. NO/DATE: IS IS/1/21/018/01-04 20210212 |
| 2317997 | 2190050-1 | Sweden | ⤷ Get Started Free | PRODUCT NAME: PHENTERMINE AND TOPIRAMATE; NAT. REG. NO/DATE: 59574-59577 20210617; FIRST REG.: IS IS/1/21/018/01-04 20210212 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for QSYMIA (Phentermine/Topiramate Extended-Release)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
