PLUVICTO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Pluvicto, and when can generic versions of Pluvicto launch?
Pluvicto is a drug marketed by Novartis and is included in one NDA. There are three patents protecting this drug.
This drug has ninety-seven patent family members in thirty-two countries.
The generic ingredient in PLUVICTO is lutetium lu-177 vipivotide tetraxetan. There are four drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the lutetium lu-177 vipivotide tetraxetan profile page.
DrugPatentWatch® Generic Entry Outlook for Pluvicto
Pluvicto will be eligible for patent challenges on March 23, 2026. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 17, 2034. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for PLUVICTO?
- What are the global sales for PLUVICTO?
- What is Average Wholesale Price for PLUVICTO?
Summary for PLUVICTO
| International Patents: | 97 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 2 |
| Drug Prices: | Drug price information for PLUVICTO |
| What excipients (inactive ingredients) are in PLUVICTO? | PLUVICTO excipients list |
| DailyMed Link: | PLUVICTO at DailyMed |
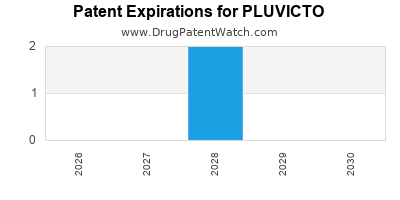

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PLUVICTO
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PLUVICTO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| VA Office of Research and Development | Phase 2 |
| OncoC4, Inc. | Phase 2 |
| Prostate Cancer Clinical Trials Consortium | Phase 2 |
Pharmacology for PLUVICTO
| Drug Class | Radioligand Therapeutic Agent |
| Mechanism of Action | Radioligand Activity |
US Patents and Regulatory Information for PLUVICTO
PLUVICTO is protected by three US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PLUVICTO is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PLUVICTO
Labeled inhibitors of prostate specific membrane antigen (PSMA), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
PSMA binding ligand-linker conjugates and methods for using
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR TREATMENT OF ADULT PATIENTS WITH PROSTATE-SPECIFIC MEMBRANE ANTIGEN (PSMA)-POSITIVE METASTATIC CASTRATION-RESISTANT PROSTATE CANCER (MCRPC) WHO HAVE BEEN TREATED WITH ANDROGEN RECEPTOR (AR) PATHWAY INHIBITION AND TAXANE-BASED CHEMOTHERAPY
PSMA binding ligand-linker conjugates and methods for using
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR TREATMENT OF ADULT PATIENTS WITH PROSTATE-SPECIFIC MEMBRANE ANTIGEN (PSMA)-POSITIVE METASTATIC CASTRATION-RESISTANT PROSTATE CANCER (MCRPC) WHO HAVE BEEN TREATED WITH ANDROGEN RECEPTOR (AR) PATHWAY INHIBITION AND TAXANE-BASED CHEMOTHERAPY
FDA Regulatory Exclusivity protecting PLUVICTO
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | PLUVICTO | lutetium lu-177 vipivotide tetraxetan | SOLUTION;INTRAVENOUS | 215833-001 | Mar 23, 2022 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | PLUVICTO | lutetium lu-177 vipivotide tetraxetan | SOLUTION;INTRAVENOUS | 215833-001 | Mar 23, 2022 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Novartis | PLUVICTO | lutetium lu-177 vipivotide tetraxetan | SOLUTION;INTRAVENOUS | 215833-001 | Mar 23, 2022 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | PLUVICTO | lutetium lu-177 vipivotide tetraxetan | SOLUTION;INTRAVENOUS | 215833-001 | Mar 23, 2022 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PLUVICTO
When does loss-of-exclusivity occur for PLUVICTO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 14336638
Patent: Labeled inhibitors of prostate specific membrane antigen (PSMA), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer
Estimated Expiration: ⤷ Sign Up
Patent: 18200419
Patent: Labeled inhibitors of prostate specific membrane antigen (PSMA), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer
Estimated Expiration: ⤷ Sign Up
Patent: 20201086
Patent: Labeled inhibitors of prostate specific membrane antigen (PSMA), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 24360
Patent: INHIBITEURS MARQUES DE L'ANTIGENE MEMBRANAIRE SPECIFIQUE DE LA PROSTATE (PSMA), LEUR UTILISATION COMME AGENTS D'IMAGERIE ET AGENTS PHARMACEUTIQUES POUR LE TRAITEMENT DU CANCER DELA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 16000883
Patent: Compuestos macrocíclicos, inhibidores de antígeno prostático específico de membrana (psma); composición farmacéutica; complejo de metal; y su uso para preparar compuestos radiomarcados útiles para la formación de imágenes para diagnosticar y para el tratamiento de cáncer de próstata y/o metástasis de la misma.
Estimated Expiration: ⤷ Sign Up
China
Patent: 5636924
Patent: Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer
Estimated Expiration: ⤷ Sign Up
Patent: 9053616
Patent: 前列腺特异性膜抗原(PSMA)的标记的抑制剂及其用途 (Labeled inhibitors of prostate specific membrane antigen (PSMA), and use thereof)
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 95130
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 7778
Patent: МЕЧЕНЫЕ ИНГИБИТОРЫ ПРОСТАТИЧЕСКОГО СПЕЦИФИЧЕСКОГО МЕМБРАННОГО АНТИГЕНА (ПСМА), ИХ ПРИМЕНЕНИЕ В КАЧЕСТВЕ АГЕНТОВ ДЛЯ ВИЗУАЛИЗАЦИИ И ФАРМАЦЕВТИЧЕСКИХ АГЕНТОВ ДЛЯ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 1690495
Patent: МЕЧЕНЫЕ ИНГИБИТОРЫ ПРОСТАТИЧЕСКОГО СПЕЦИФИЧЕСКОГО МЕМБРАННОГО АНТИГЕНА (ПСМА), ИХ ПРИМЕНЕНИЕ В КАЧЕСТВЕ АГЕНТОВ ДЛЯ ВИЗУАЛИЗАЦИИ И ФАРМАЦЕВТИЧЕСКИХ АГЕНТОВ ДЛЯ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 62857
Patent: Inhibiteurs marqués de l'antigène membranaire spécifique de la prostate (PSMA), leur utilisation comme agents d'imagerie et agents pharmaceutiques pour le traitement du cancer de la prostate (Labeled inhibitors of prostate specific membrane antigen (PSMA), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer)
Estimated Expiration: ⤷ Sign Up
Patent: 38996
Patent: INHIBITEURS MARQUÉS DE L'ANTIGÈNE MEMBRANAIRE SPÉCIFIQUE DE LA PROSTATE (PSMA), LEUR UTILISATION COMME AGENTS D'IMAGERIE ET AGENTS PHARMACEUTIQUES POUR LE TRAITEMENT DU CANCER DE LA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 15489
Patent: INHIBITEURS MARQUÉS DE L'ANTIGÈNE MEMBRANAIRE SPÉCIFIQUE DE LA PROSTATE (PSMA), LEUR UTILISATION COMME AGENTS D'IMAGERIE ET AGENTS PHARMACEUTIQUES POUR LE TRAITEMENT DU CANCER DE LA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 56700
Patent: INHIBITEURS MARQUÉS DE L'ANTIGÈNE MEMBRANAIRE SPÉCIFIQUE DE LA PROSTATE (PSMA), LEUR UTILISATION COMME AGENTS D'IMAGERIE ET AGENTS PHARMACEUTIQUES POUR LE TRAITEMENT DU CANCER DE LA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 95355
Patent: INHIBITEURS MARQUÉS DE L'ANTIGÈNE MEMBRANAIRE SPÉCIFIQUE DE LA PROSTATE (PSMA), LEUR UTILISATION COMME AGENTS D'IMAGERIE ET AGENTS PHARMACEUTIQUES POUR LE TRAITEMENT DU CANCER DE LA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 95130
Patent: INHIBITEURS MARQUÉS DE L'ANTIGÈNE MEMBRANAIRE SPÉCIFIQUE DE LA PROSTATE (PSMA), LEUR UTILISATION COMME AGENTS D'IMAGERIE ET AGENTS PHARMACEUTIQUES POUR LE TRAITEMENT DU CANCER DE LA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 95130
Estimated Expiration: ⤷ Sign Up
Georgia, Republic of
Patent: 0217330
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Patent: 0237479
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Patent: 0237496
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Patent: 0237497
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Germany
Patent: 2014011593
Estimated Expiration: ⤷ Sign Up
Patent: 2014011600
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 21711
Patent: 前列腺特異性膜抗原 的標記的抑制劑,它們作為顯影劑和用於治療前列腺癌的藥劑的用途 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER (PSMA))
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 5113
Patent: מעכבים מסומנים של אנטיגן ממברנה ספציפי לערמונית (psma), השימוש בהם כחומרי הדמיה וכחומרי רוקחות לטיפול בסרטן הערמונית (Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer)
Estimated Expiration: ⤷ Sign Up
Patent: 8974
Patent: מעכבים מסומנים של אנטיגן ממברנה ספציפי לערמונית (psma), השימוש בהם כחומרי הדמיה וכחומרי רוקחות לטיפול בסרטן הערמונית (Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer)
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 56805
Estimated Expiration: ⤷ Sign Up
Patent: 01451
Estimated Expiration: ⤷ Sign Up
Patent: 36774
Estimated Expiration: ⤷ Sign Up
Patent: 94161
Estimated Expiration: ⤷ Sign Up
Patent: 93485
Estimated Expiration: ⤷ Sign Up
Patent: 16535013
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用
Estimated Expiration: ⤷ Sign Up
Patent: 18058847
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 19011368
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用 (LABEL INHIBITOR OF PROSTATE-SPECIFIC MEMBRANE ANTIGEN (PSMA), AND APPLICATION THEREOF AS IMAGING AGENT AND PHARMACEUTICAL FOR TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 19218351
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), AND THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 21059557
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 22159345
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用
Estimated Expiration: ⤷ Sign Up
Patent: 24028742
Patent: 前立腺特異的膜抗原(PSMA)の標識インヒビター、前立腺癌の治療のための画像化剤および薬剤としてのその使用
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 95130
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 8934
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Patent: 4484
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 16005013
Patent: INHIBIDORES MARCADOS DE ANTIGENO PROSTATICO ESPECIFICO DE MEMBRANA (PSMA), SU USO COMO AGENTES FORMADORES DE IMAGENES Y AGENTES FARMACEUTICOS PARA EL TRATAMIENTO DE CANCER DE PROSTATA. (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER.)
Estimated Expiration: ⤷ Sign Up
Patent: 21008976
Patent: INHIBIDORES MARCADOS DE ANTIGENO PROSTATICO ESPECIFICO DE MEMBRAMA (PSMA), SU USO COMO AGENTES FORMADORES DE IMAGENES Y AGENTES FARMACEUTICOS PARA EL TRATAMIENTO DE CANCER DE PROSTATA. (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER.)
Estimated Expiration: ⤷ Sign Up
Patent: 21008977
Patent: INHIBIDORES MARCADOS DE ANTIGENO PROSTATICO ESPECIFICO DE MEMBRAMA (PSMA), SU USO COMO AGENTES FORMADORES DE IMAGENES Y AGENTES FARMACEUTICOS PARA EL TRATAMIENTO DE CANCER DE PROSTATA. (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER.)
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 986
Patent: Inhibiteurs marqués de l'antigène membranaire spécifique de la prostate (psma), leur utilisation comme agents d'imagerie et agents pharmaceutiques pour le traitement du cancer de la prostate
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 8812
Patent: Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 160678
Patent: INHIBIDORES MARCADOS DE ANTIGENO PROSTATICO ESPECIFICO DE MEMBRANA (PSMA), SU USO COMO AGENTES FORMADORES DE IMAGENES Y AGENTES FARMACEUTICOS PARA EL TRATAMIENTO DE CANCER DE PROSTATA
Estimated Expiration: ⤷ Sign Up
Patent: 211760
Patent: INHIBIDORES MARCADOS DE ANTIGENO PROSTATICO ESPECIFICO DE MEMBRANA (PSMA) QUE COMPRENDEN GRUPOS CARBOXILICOS Y UNA REGION DE ENLAZADOR MODIFICADA, AGENTES FORMADORES DE IMAGENES Y AGENTES FARMACEUTICOS QUE LOS COMPRENDEN
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 016500656
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 95130
Estimated Expiration: ⤷ Sign Up
Saudi Arabia
Patent: 6370842
Patent: مثبطات مصنفة لمُستضد غشاء معين للبروتستاتا (PSMA)، استخدامها كعوامل تصوير وكعوامل صيدلانية لعلاج سرطان البروستاتا (Labeled Inhibitors of Prostate Specific Membrane Antigen (PSMA), Their Use as Imaging Agents and Pharmaceutical Agents for the Treatment of Prostate Cancer)
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 324
Patent: OBELEŽENI INHIBITORI MEMBRANSKOG ANTIGENA SPECIFIČNOG ZA PROSTATU (PSMA), NJIHOVA UPOTREBA KAO AGENASA ZA SNIMANJE I FARMACEUTSKIH AGENASA ZA LEČENJE KARCINOMA PROSTATE (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201602249R
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1603380
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
Patent: 1907607
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1947053
Estimated Expiration: ⤷ Sign Up
Patent: 2210931
Estimated Expiration: ⤷ Sign Up
Patent: 2282378
Estimated Expiration: ⤷ Sign Up
Patent: 160063398
Patent: 전립선 특이적 막 항원(PSMA)의 표지된 억제제, 조영제로서의 이의 용도 및 전립선암 치료용 약제 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 190016133
Patent: 전립선 특이적 막 항원(PSMA)의 표지된 억제제, 조영제로서의 이의 용도 및 전립선암 치료용 약제 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN PSMA THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Patent: 210013350
Patent: 전립선 특이적 막 항원(PSMA)의 표지된 억제제, 조영제로서의 이의 용도 및 전립선암 치료용 약제 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN PSMA THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER)
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 16000137
Patent: LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER.
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PLUVICTO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | 2021008977 | INHIBIDORES MARCADOS DE ANTIGENO PROSTATICO ESPECIFICO DE MEMBRAMA (PSMA), SU USO COMO AGENTES FORMADORES DE IMAGENES Y AGENTES FARMACEUTICOS PARA EL TRATAMIENTO DE CANCER DE PROSTATA. (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER.) | ⤷ Sign Up |
| Hungary | E047200 | ⤷ Sign Up | |
| Chile | 2016000883 | Compuestos macrocíclicos, inhibidores de antígeno prostático específico de membrana (psma); composición farmacéutica; complejo de metal; y su uso para preparar compuestos radiomarcados útiles para la formación de imágenes para diagnosticar y para el tratamiento de cáncer de próstata y/o metástasis de la misma. | ⤷ Sign Up |
| South Africa | 201907607 | LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA), THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER | ⤷ Sign Up |
| South Korea | 20210013350 | 전립선 특이적 막 항원(PSMA)의 표지된 억제제, 조영제로서의 이의 용도 및 전립선암 치료용 약제 (LABELED INHIBITORS OF PROSTATE SPECIFIC MEMBRANE ANTIGEN PSMA THEIR USE AS IMAGING AGENTS AND PHARMACEUTICAL AGENTS FOR THE TREATMENT OF PROSTATE CANCER) | ⤷ Sign Up |
| China | 105636924 | Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer | ⤷ Sign Up |
| Israel | 245113 | מעכבים מסומנים של אנטיגן ממברנה ספציפי לערמונית (psma), השימוש בהם כחומרי הדמיה וכחומרי רוקחות לטיפול בסרטן הערמונית (Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


