PATADAY Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Pataday, and when can generic versions of Pataday launch?
Pataday is a drug marketed by Alcon Labs Inc and is included in three NDAs. There are two patents protecting this drug.
The generic ingredient in PATADAY is olopatadine hydrochloride. There are seventeen drug master file entries for this compound. Forty-five suppliers are listed for this compound. Additional details are available on the olopatadine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Pataday
A generic version of PATADAY was approved as olopatadine hydrochloride by APOTEX INC on October 8th, 2014.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PATADAY?
- What are the global sales for PATADAY?
- What is Average Wholesale Price for PATADAY?
Summary for PATADAY
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 3 |
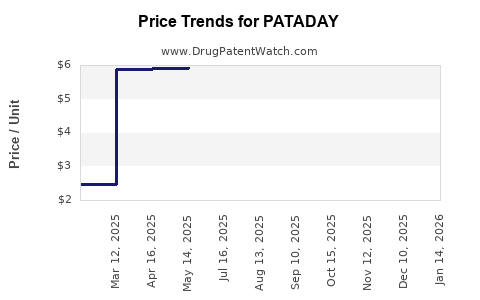
| Drug Prices: | Drug price information for PATADAY |
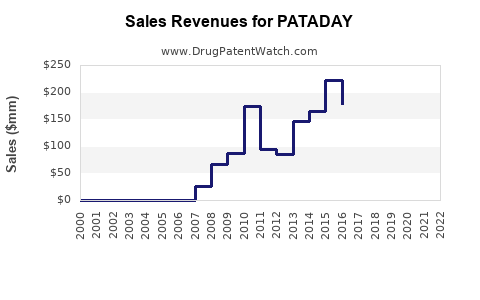
| Drug Sales Revenues: | Drug sales revenues for PATADAY |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PATADAY |
| What excipients (inactive ingredients) are in PATADAY? | PATADAY excipients list |
| DailyMed Link: | PATADAY at DailyMed |
Recent Clinical Trials for PATADAY
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Andover Research Eye Institute | Phase 4 |
| Allergan | Phase 4 |
| McCabe Vision Center | N/A |
US Patents and Regulatory Information for PATADAY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | PATADAY ONCE DAILY RELIEF | olopatadine hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 021545-001 | Dec 22, 2004 | OTC | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Alcon Labs Inc | PATADAY TWICE DAILY RELIEF | olopatadine hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 020688-001 | Dec 18, 1996 | OTC | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Alcon Labs Inc | PATADAY ONCE DAILY RELIEF | olopatadine hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 206276-001 | Jan 30, 2015 | OTC | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Alcon Labs Inc | PATADAY ONCE DAILY RELIEF | olopatadine hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 206276-001 | Jan 30, 2015 | OTC | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
