ONPATTRO Drug Patent Profile
✉ Email this page to a colleague
When do Onpattro patents expire, and when can generic versions of Onpattro launch?
Onpattro is a drug marketed by Alnylam Pharms Inc and is included in one NDA. There are fourteen patents protecting this drug.
This drug has two hundred and fifty-one patent family members in thirty-one countries.
The generic ingredient in ONPATTRO is patisiran sodium. One supplier is listed for this compound. Additional details are available on the patisiran sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Onpattro
Onpattro was eligible for patent challenges on August 10, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 10, 2032. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ONPATTRO?
- What are the global sales for ONPATTRO?
- What is Average Wholesale Price for ONPATTRO?
Summary for ONPATTRO
| International Patents: | 251 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 1 |
| Drug Prices: | Drug price information for ONPATTRO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ONPATTRO |
| What excipients (inactive ingredients) are in ONPATTRO? | ONPATTRO excipients list |
| DailyMed Link: | ONPATTRO at DailyMed |
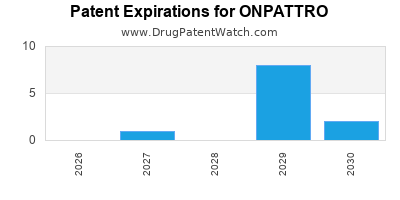

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ONPATTRO
Generic Entry Date for ONPATTRO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ONPATTRO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Alnylam Pharmaceuticals | Early Phase 1 |
| Austin Neuromuscular Center | Early Phase 1 |
Pharmacology for ONPATTRO
| Drug Class | Small Interfering RNA Transthyretin-directed RNA Interaction |
| Physiological Effect | Decreased RNA Integrity Increased Protein Breakdown |
US Patents and Regulatory Information for ONPATTRO
ONPATTRO is protected by fourteen US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ONPATTRO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ONPATTRO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ONPATTRO
When does loss-of-exclusivity occur for ONPATTRO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09307677
Estimated Expiration: ⤷ Get Started Free
Patent: 15249072
Estimated Expiration: ⤷ Get Started Free
Patent: 17225110
Estimated Expiration: ⤷ Get Started Free
Patent: 19216630
Estimated Expiration: ⤷ Get Started Free
Patent: 21203272
Estimated Expiration: ⤷ Get Started Free
Patent: 23248138
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0919732
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 39895
Estimated Expiration: ⤷ Get Started Free
Patent: 18487
Estimated Expiration: ⤷ Get Started Free
Patent: 22620
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2186978
Estimated Expiration: ⤷ Get Started Free
Patent: 3937793
Estimated Expiration: ⤷ Get Started Free
Patent: 6834291
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150796
Estimated Expiration: ⤷ Get Started Free
Patent: 0180093
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16944
Estimated Expiration: ⤷ Get Started Free
Patent: 20174
Estimated Expiration: ⤷ Get Started Free
Patent: 19005
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 44639
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0312
Estimated Expiration: ⤷ Get Started Free
Patent: 9762
Estimated Expiration: ⤷ Get Started Free
Patent: 6772
Estimated Expiration: ⤷ Get Started Free
Patent: 1170591
Estimated Expiration: ⤷ Get Started Free
Patent: 1400170
Estimated Expiration: ⤷ Get Started Free
Patent: 1792626
Estimated Expiration: ⤷ Get Started Free
Patent: 2092118
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 44639
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
Patent: 54733
Estimated Expiration: ⤷ Get Started Free
Patent: 48461
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 56348
Estimated Expiration: ⤷ Get Started Free
Patent: 16652
Estimated Expiration: ⤷ Get Started Free
Patent: 58859
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 26604
Estimated Expiration: ⤷ Get Started Free
Patent: 37875
Estimated Expiration: ⤷ Get Started Free
Patent: 900004
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3178
Estimated Expiration: ⤷ Get Started Free
Patent: 1434
Estimated Expiration: ⤷ Get Started Free
Patent: 2142
Estimated Expiration: ⤷ Get Started Free
Patent: 0600
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 91505
Estimated Expiration: ⤷ Get Started Free
Patent: 71446
Estimated Expiration: ⤷ Get Started Free
Patent: 53780
Estimated Expiration: ⤷ Get Started Free
Patent: 00658
Estimated Expiration: ⤷ Get Started Free
Patent: 12506254
Estimated Expiration: ⤷ Get Started Free
Patent: 15062420
Estimated Expiration: ⤷ Get Started Free
Patent: 18007666
Estimated Expiration: ⤷ Get Started Free
Patent: 18171072
Estimated Expiration: ⤷ Get Started Free
Patent: 19205442
Estimated Expiration: ⤷ Get Started Free
Patent: 22160408
Estimated Expiration: ⤷ Get Started Free
Patent: 24116226
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 937418
Estimated Expiration: ⤷ Get Started Free
Patent: 2019501
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0098
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 4354
Estimated Expiration: ⤷ Get Started Free
Patent: 0460
Estimated Expiration: ⤷ Get Started Free
Patent: 11004268
Estimated Expiration: ⤷ Get Started Free
Patent: 18013398
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 0965
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2867
Patent: COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF TRANSTHYRETIN
Estimated Expiration: ⤷ Get Started Free
Patent: 2404
Patent: Compositions and methods for inhibiting expression of transthyretin
Estimated Expiration: ⤷ Get Started Free
Patent: 8858
Patent: Compositions and methods for inhibiting expression of transthyretin
Estimated Expiration: ⤷ Get Started Free
Patent: 7298
Patent: Compositions and methods for inhibiting expression of transthyretin
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 19004
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 44639
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 44639
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500176
Patent: Composizioni e metodi per inibire l'espressione ditranstiretina
Estimated Expiration: ⤷ Get Started Free
Patent: 01800027
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201703242X
Patent: COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF TRANSTHYRETIN
Estimated Expiration: ⤷ Get Started Free
Patent: 201809460S
Patent: COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF TRANSTHYRETIN
Estimated Expiration: ⤷ Get Started Free
Patent: 201912276X
Patent: COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF TRANSTHYRETIN
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 44639
Estimated Expiration: ⤷ Get Started Free
Patent: 37418
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1102876
Patent: COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF TRANSTHYRETIN
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1624869
Estimated Expiration: ⤷ Get Started Free
Patent: 1635436
Estimated Expiration: ⤷ Get Started Free
Patent: 2031027
Estimated Expiration: ⤷ Get Started Free
Patent: 2354558
Estimated Expiration: ⤷ Get Started Free
Patent: 2578331
Estimated Expiration: ⤷ Get Started Free
Patent: 110073592
Estimated Expiration: ⤷ Get Started Free
Patent: 150007359
Estimated Expiration: ⤷ Get Started Free
Patent: 160079921
Estimated Expiration: ⤷ Get Started Free
Patent: 180017245
Estimated Expiration: ⤷ Get Started Free
Patent: 190116553
Estimated Expiration: ⤷ Get Started Free
Patent: 220012422
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 43004
Estimated Expiration: ⤷ Get Started Free
Patent: 56516
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ONPATTRO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Portugal | 2028278 | ⤷ Get Started Free | |
| Norway | 333713 | ⤷ Get Started Free | |
| Australia | 3574402 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ONPATTRO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2937418 | 2019004 | Norway | ⤷ Get Started Free | PRODUCT NAME: PATISIRAN: DEKKET GJENNOM SEKVENSENE I KRAVENE 1, 8 OG 9 (SE SAERLIG KRAV 8 (B) OG (C) I PATENTET. DET VISES OGSA TIL FOELGEBREVET TIL SOEKNADEN.; REG. NO/DATE: EU/1/18/1320 20180911 |
| 2937418 | SPC/GB19/005 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: PATISIRAN; REGISTERED: UK EU/1/18/1320(FOR NI) 20180827; UK PLGB 50597/0002 20180827 |
| 2937418 | PA2019501,C2937418 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: PATISIRANAS; REGISTRATION NO/DATE: EU/1/18/1320 20180827 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ONPATTRO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
